NAZAL VE PARANAZAL SİNÜS CERRAHİSİNDE UYGULANAN YARA İYİLEŞTİRİCİ AJANLARIN ETKİLERİ
2Süleyman Demirel Üniversitesi Tıp Fakültesi , Patoloji Anabilim Dalı, Isparta, Türkiye
Özet
Amaç: Burun kanamasını durdurmada veya burun cerrahisi sonrasında kullanılan burun tampon materyalleri, parafin veya antibiyotikli krem ve/veya pomatlar gibi nemlendirici ve kayganlaştırıcı maddelerle hazırlanırlar. Bu çalışmanın amacı yara iyileştirici ajanların burun tamponu hazırlanmasında kullanılıp kullanılamayacağını araştırmaktır.Gereç ve Yöntemler: Bu çalışmada; 48 adet erkek, albino, Yeni Zelanda tavşanı kullanıldı. Nazal septum ve lateral nazal duvarda mukozal hasar oluşturulduktan sonra nazal kavitelere yumuşak parafin, clostridiopeptidase-A, nitrofurazon ve asiaticoside’li kremler uygulandı. Yara iyileşmesinin erken ve geç dönemlerindeki etkileri histopatolojik olarak incelendi.
Bulgular: Asiaticoside ve clostridiopeptidase-A erken dönem mukozal ödemin ve siliya kaybının önlenmesinde nitrofurazon ve kontrol gruplarından daha iyi bulunmuştur (p<0.05).
Sonuç: Yara iyileştirici ajan olan asiaticoside burun içinde etkinliği birbirine yakın olan clostridiopeptidase-A ve nitrofurazondan daha faydalı bulunmuştur.
Giriş
Wound healing is a highly complex, coordinated, multistep process involving clot formation, inflammatory reaction, immune response, and finally, tissue remodeling and maturation. Many endogenous and exogenous influences, such as radiation, infection, nutrition, systemic factors, and surgical technique affect this process.[1,2] Surgical wounds secondary to some interventions and some disorders such as epistaxis due to various factors necessitate mostly nasal packing. A moist wound environment promotes wound healing, accelerates epithelization and suppresses the inflammatory reaction and development of necrosis in the early stage and scar formation in the late stage.[3] Hence, nasal packing materials are impregnated with some ointments in order to prevent drying and crusting of mucosa, clotting, and to minimize the discomfort created by dry packing.[4] Our study was aimed to evaluate alternative agents in nasal packing impregnation, at the same time promoting the healing process. For this purpose we preferred to use wound healing agents in an experimental study in preventing possible unfavourable effects of routinely used methods.The formulations and ointments investigated in this study have been selected that are available on the pharmacies in Turkey, and no support was taken from the manufacturers.
Yöntem ve Gereçler
Forty eight Pasteurella-free New Zealand white rabbits of male sex were used for this study each weighing between 3.5 and 5.5 kilograms. Permission was obtained from Süleyman Demirel University Medical Ethic Board for all experimental methods. The animals were cared for under standardized conditions for a period of 3 weeks before surgery. They had access to pelleted food and water throughout the experiment. All animals were anesthetized with ketamine 35 mg/kg IM, Xylazine 5 mg/kg IM.Forty eight rabbits were divided mainly into two groups (group A and group B); and then each of groups were subdivided into four subgroups. Each of Group A and group B were composed of 4 subgroups of; 6 clostridiopeptidase-A (Novuxol® Knoll), 6 asiaticoside (Madecassol® Roche Consumer Health), 6 nitrofurazone (Furacin® Procter & Gamble) and 6 control (soft paraffine) (Table.1).
Table 1: The number of rabbits in group A and group B distributed in subgroups
Surgical technique:
The nasal dorsum was shaved and prepped with betadine and draped in a sterile fashion. An incision in the midline of the nasal dorsum was made through the skin, subcutaneous tissue and periosteum of the nasal bones, and the skin flaps were elevated and retracted laterally. A rotating burr was used to remove approximately 6 mm of bone from both nasal bones bilaterally to access the nasal cavity. Nasal cavity was inspected with a 2.7 mm 0 and 30 degree rigid sinus telescopes (Karl Storz, Hopkins, Germany). Mucosae of nasal septum and latarel nasal walls of both nasal fossae were incised with a microblade from choanae towards nostrils. During that procedure care was taken to avoid the septal cartilage injury. Then the control agent (soft paraffin) or experimental ointments were administered bilaterally into each nasal cavity by one investigator. The skin incision was sutured with 3/0 sterile coated vicryl. No systemic medication was administered. Sterility was maintained throughout the procedure.
Histologic Techniques
Rabbits of group A and of group B were sacrificed after 7 and 21 days of surgical intervention respectively. Ten cc of a saturated solution of potassium chloride was administered via intracardiac injection to kill the rabbits. The head was removed, soft tissue trimmed, and the skull placed in 10 % neutral buffered formalin. After formalin fixation, a tissue specimen comprised of the sinuses and nasal septum was prepared. The tissue specimens were decalcified in decalcifying solution Christiensen’s fluid (equal volumes of 40% formic acid and 6.8% sodium formiate) until softened at room temperature. The sinuses and nasal septum were dissected in coronal plane, with the intervals of 4 mm, from choanae to nares. Then, the removed segments of tissues were embedded in paraffin. Light microscopy slides were prepared from the specimens stained with hematoxylin-eosine and Masson’s trichrome. The slides were labelled randomly by the investigator and presented to the pathologist for interpretation. The slides were evaluated in blinded fashion and differentiated according to histopathologic findings. The histopathologic examination was performed, blindly, regarding the number of goblet cells (Fig. 1), mucosal edema (Fig. 2), cartilage degeneration of nasal septum (Fig. 3), degree of ciliary loss in nasal epithelium (Fig. 4), lipogranulomatous reaction (Fig. 1), intranasal synechia (Fig. 5), squamous cell metaplasia of nasal epithelium, fibrosis of subepithelial connective tissue by light microscopy (Nikon Optiphod-B, Japan).
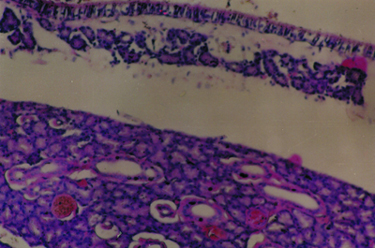 Büyütmek İçin Tıklayın |
Fig 1: Magnification of turbinate showing goblet cell hyperplasia, mucous retention cysts and lipogranulomatous reaction (hematoxylin-eosin, original magnification X 200). |
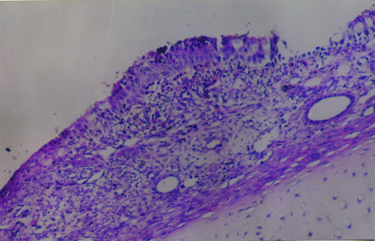 Büyütmek İçin Tıklayın |
Fig 2: Magnification of nasal septum showing mucosal thickening due to mucosal edema and inflammatory cell infiltration (hematoxylin-eosin, original magnification X 100). |
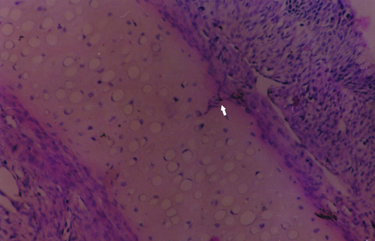 Büyütmek İçin Tıklayın |
Fig 3: Magnification of nasal septum showing cartilage degeneration (hematoxylin-eosin, original magnification X 100). |
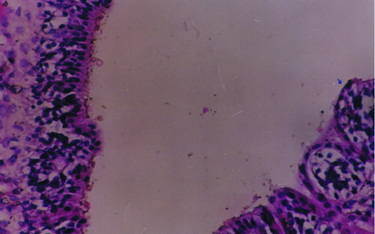 Büyütmek İçin Tıklayın |
Fig 4: Magnification of turbinate showing ciliated columnar epithelium (A) and ciliary loss (B) (hematoxylin-eosin, original magnification X 200). |
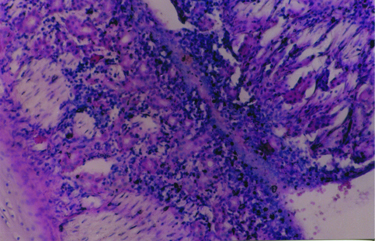 Büyütmek İçin Tıklayın |
Fig 5: Magnification of nasal septum and turbinate showing intranasal synechia; vascular proliferation is prominent between two structures (hematoxylin-eosin, original magnification X 100). |
These histopathologic parameters were graded semiquantitatively as normal (0), mild (1), moderate (2), or severe (3). Statistical evaluation was performed by using the SPSS software program (Fisher exact test).
Bulgular
At the end of first week of manipulation (early phase of wound healing) histopathologic findings of tissue specimens which are belonging to group A (Table. 2) were as follows:Mucosal edema was determined in 1/6 of clostridiopeptidase-A subgroup, 1/6 of asiaticoside subgroup, 4/6 of nitrofurazone subgroup and all of the control subgroup. Mucosal edema was significantly reduced in clostridiopeptidase-A and asiaticoside subgroups compared to those of nitrofurazone and control subgroups during early stage of wound healing (p<0.05).
Goblet cell hyperplasia was determined in 3/6 of clostridiopeptidase-A subgroup, 3/6 of asiaticoside subgroup, 2/6 of nitrofurazone subgroup and 3/6 of the control subgroup. Cartilage degeneration of nasal septum was determined in only 1/6 of nitrofrazone subgroup. Degree of ciliary loss in nasal epithelium was determined in 2/6 of clostridiopeptidase-A subgroup, 1/6 of asiaticoside subgroup, 2/6 of nitrofurazone subgroup and 2/6 of the control subgroup. Lipogranulomatous reaction was not determined in any of the group A. Intranasal synechia fomation was determined in only 1/6 of nitrofrazone subgroup. Squamous cell metaplasia of nasal epithelium was determined in 1/6 of clostridiopeptidase-A subgroup, 1/6 of asiaticoside subgroup, 3/6 of nitrofurazone subgroup and 3/6 of the control subgroup. Degree of subepithelial firosis was determined in 4/6 of clostridiopeptidase-A subgroup, 3/6 of asiaticoside subgroup, 5/6 of nitrofurazone subgroup and 5/6 of the control subgroup.
There was no statistically significant difference among the subgroups of group A regarding goblet cell hyperplasia, cartilage degeneration of nasal septum, degree of ciliary loss in nasal epithelium, lipogranulomatous reaction, intranasal synechia fomation, squamous cell metaplasia of nasal epithelium and the degree of subepithelial firosis.
At 21st day of manipulation (late phase of wound healing) histopathologic findings of tissue specimens which are belonging to group B (Table. 2) were as follows:
Goblet cell hyperplasia was determined in 5/6 of clostridiopeptidase-A subgroup, 1/6 of asiaticoside subgroup, 5/6 of nitrofurazone subgroup and 0/6 of the control subgroup. Goblet cell hyperplasia was significantly higher in clostridiopeptidase-A and nitrofurazone groups than asiaticoside and control groups (p<0.05).
Degree of ciliary loss in nasal epithelium was determined in 2/6 of nitrofurazone, 3/6 of the control and none of clostridiopeptidase-A and asiaticoside subgroups. Degree of ciliary loss in nasal epithelium was significantly higher in nitrofurazone and control subgroups compared to those of clostridiopeptidase-A and asiaticoside subgroups during late stage of wound healing (p<0.05).
Mucosal edema was determined in 2/6 of clostridiopeptidase-A subgroup, 1/6 of asiaticoside subgroup, 2/6 of nitrofurazone subgroup and 3/6 of the control subgroup. Cartilage degeneration of nasal septum was determined in 1/6 of clostridiopeptidase-A subgroup, 1/6 of nitrofurazone subgroup and none of asiaticoside and control subgroups. Lipogranulomatous reaction was determined in 1/6 of clostridiopeptidase-A subgroup, 2/6 of the control subgroup and none of asiaticoside and nitrofurazone subgroups. Intranasal synechia formation was not determined in any of subgroups of group B. Squamous cell metaplasia of nasal epithelium was determined in 1/6 of nitrofurazone, 3/6 of the control subgroups and none of clostridiopeptidase-A and asiaticoside subgroups. Degree of subepithelial fibrosis was determined in 4/6 of clostridiopeptidase-A subgroup, 1/6 of asiaticoside subgroup, 2/6 of nitrofurazone subgroup and 3/6 of the control subgroup.
There was no statistically significant difference among the subgroups of group B regarding mucosal edema, cartilage degeneration of nasal septum, lipogranulomatous reaction, intranasal synechia formation, squamous cell metaplasia of nasal epithelium and the degree of subepthelial fibrosis.
Tartışma
Packing the nose after all types of nasal surgery and as an intervention for epistaxis is a method of controlling bleeding.[5] Nasal packing for the first few days after septal surgery is intended to prevent swelling and formation of septal hematoma postoperatively.[6] Placement and removal of nasal packing may create mild to moderate degree of wound model in the nasal mucosae. Nasal packing materials are impregnated with some ointments in order to prevent drying and crusting of mucosa, clotting, and to minimize the discomfort created by dry packing.[4] In our clinic, besides the septal surgery, we also insert a small ribbon gauze impregnated with ointment between the middle turbinate and maxillary ostium just after the interventions to the ipsilateral sinuses to prevent synechia formation between them. By this way synechia formation especially between middle turbinate and lateral nasal wall, so the obstruction of middle meatus, will be prevented. It is important because the obstruction of ostia of sinuses plays a role in the pathogenesis of chronic sinusitis.[7]McIntosh et al.[8] investigated the effect of dissolvable hyaluronic acid-based pack on the healing of the nasal mucosa of sheep and found that, dissolvable hyaluronic acid-based pack improved reepithelization. Soldati et al.[4] investigated the efficacy and tolerability of hyaluronic acid in the form of a 0.2% nasal cream in a clinical study, and found that; it prevents the crusting and improves the nasal airflow. Kehrl et al.[9] demonstrated the beneficial effects of dexpanthenol and xylomethazoline combination for intranasal wound healing. We investigated the efficacy and tolerability of different wound healing agents, which are cheaper and easier to find, both for nasal surgical intervention and for some manipulation such as nasal packing due to epistaxis.
Wound healing relies on the integration and coordination of many cellular and humeral elements.[2] Wound healing necessitates special wound care in different parts of the body and in different wound types, mostly requiring carefully regulated, spatially organized degradation of collagen. Endogenous collagenases perform an important function to clear wounds of proteinacous debris. Tissue remodelling phase of wound healing is affected by decreased collagen and ground substance synthesis.[10] Clostridiopeptidase-A, an enzyme preparation derived from Clostridium histolyticum, digests both native and denatured collagen.[11] In a study conducted by Mekkes et al.[12] wound debriding properties of collagenase ointment and speed of epithelization were better than fibrinolysine/DNAse oleogel. Damages to newly formed epithelium at the wound margins, maceration and desquamation, possible side effects of proteolytic enzymes, didn’t seem after administration of collagenase ointment.[12] In our study it was demonstrated that clostridiopeptidase-A reduces the mucosal edema and prevents damage to ciliary structures.
In the excision-type of wound healing, enzymatic and non-enzymatic antioxidants remain depleted and they may or may not recover following the completion of healing.[13] Depleted levels of various antioxidants may contribute to delayed healing.[14] Asioticoside derived from Centella asiatica promote healing in both normal and delayed healing type wounds by promoting collagen synthesis and angiogenesis.[15] In order to carry out these effects asiaticoside augments both enzymatic (reduced glutathion, vitamin C and vitamin E) and non-enzymatic (superoxide dismutase, catalase and glutathionperoxidase), and decreases malondialdehyde which is end product of lipid peroxidation, especially during early phase of wound healing.[14]
Goblet cell formation is a host response to the infection. Increased mucus production would physically coat and protect the mucosa from the bacteria and inflammatory mediators in the lumen.[16] In our study goblet cell hyperplasia was interestingly higher in clostridiopeptidase-A and nitrofurazone subgroups than control and asiaticoside subgroups. This was probably because of reaction of the tissue to the ointment rather than infection. Since there was no prominent inflammatory cell infiltration in subepithelial tissue, there were some foreign body-type reactions.
Myospherulosis, developed from an emulsion between erythrocytes and petrolatum or lipid products, is a foreign body type reaction that creates accumulations of erythrocyte-containing saccule.[17] Kakizaki et al.[18] reproduced myospherulosis in all cases by mixing erythrocytes in vitro with vitamin E, oleic acid, linoleic acid, lanolin. We have detected foreign body type reaction in two of paraffin subgroups and in only one of clostridiopeptidase-A.
In our study, asiaticoside had remarkable beneficial effects on nasal wound healing process regarding mucosal edema, subepithelial fibrosis, ciliary loss, and hyperplasia of goblet cells. Clostridiopeptidase-A had preventive effects against the development of mucosal edema during early phase, and ciliary loss during the late phase of wound healing. But at the same time it had unfavourable effect such as goblet cell hyperplasia during the late phase. Nitrofurazone had unfavourable effects such as mucosal edema during early phase, and goblet cell hyperplasia and ciliary loss during late phase of wound healing. In the lights of these findings we suppose that; clostridiopeptidase-A and asiaticoside can be used in nasal surgery, and in nasal packing for epistaxis. These two agents were as useful as at least nitrofurazone.
Sonuç
Wound healing agent asiaticoside has more beneficial effects on nasal packing and nasal surgery than clostridiopeptidase-A and nitrofurazone. Clostridiopeptidase-A has nearly the similar effects with that of nitrofurazonee, and can be preferred as an alternative agent. Further clinical research is needed to get better insight in the exact effects of various wound healing agents on ciliary functions and nasal airflow.Kaynaklar
1) Watelet JB, Bachert C, Gevaert P, Van Cauwenberge P. Wound healing of the nasal and paranasal mucosa: a review. Am J Rhinol 2002; 16:77-84. [ Özet ]
2) Brown MT: Wound Healing; In Otolaryngology Head & Neck Surgery. 3rd ed. Cummings CW, Frederickson JM, Harker LA, Krause CJ, Richardson MA, Schüller DE (Eds): St.Louis, Mosby, 187-196, 1998
3) Weber R, Keerl R, Hoshapfel F, Draf W, Toffel PH. Packing in endonasal surgery. Am J Otolaryngol 2001; 22:306-320. [ Özet ]
4) Soldati D, Rahm F, Pasche P. Mucosal wound healing after nasal surgery. A controlled clinical trial on the efficacy of hyaluronic acid containing cream. Drugs Exp Clin Res 1999; 25:253-261. [ Özet ]
5) Laing MR, Clark LJ. Analgesia and removal of nasal packing. Clin Otolaryngol 1990; 50:339-342. [ Özet ]
6) Illum P, Grymer L, Hilberg O. Nasal packing after septoplasty. Clin Otolaryngol 1992; 17:158-162. [ Özet ]
7) Hassab MH, Kennedy DW. Effects of long-term induced ostial obstruction in the rabbit maxillary sinus. Am J Rhinol 2001; 15:55-59. [ Özet ]
8) McIntosh D, Cowin A, Adams D, Rayner T, Wormald PJ. The effect of a dissolvable hyaluronic acid-based pack on the healing of the nasal mucosa of sheep. Am J Rhinol 2002; 16:85-90. [ Özet ]
9) Kehrl W, Sonnemann U. Improving wound healing after nose surgery by combined administration of xylometazoline and dexpanthenol. Laryngorhinootologie 2000; 79;151-154. [ Özet ]
10) Karunkoda SR, Flynn TC, Boh EE, McBurney EI, Russo GG, Millikan LE. The effects of drugs on wound healing-part II. Specific classes of drugs and their effect on healing wounds. Int J Dermatol 2000; 39:321-333. [ Özet ]
11) Hansbrough JF, Hansbrough W. Enzymatic debridement of burn wounds. Wounds 1995; 7:228-233.
12) Mekkes JR, Zeegelaar JE, Wetsrhof W. Quantitative and objective evaluation of wound debriding properties of collagenase and fibrynolysin/desoxyribonuclease in a necrotic ulcer animal model. Arch Dermatol Res 1998; 290:152-157. [ Özet ]
13) Shukla A, Rasik AM, Patnaik GK. Depletion of reduced glutathion, ascorbic acid, vitamin E and anti-oxidant defence enzymes in a healing cutaneous wound. Free Radical Res 1997; 26:93-101. [ Özet ]
14) Shukla A, Rasik AM, Dhawan BN. Asioticoside-induced elevation of antioxidant levels in healing wounds. Phytotherapy Research 1999; 13:50-54. [ Özet ]
15) Shukla A, Rasik AM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN. In vitro and in vivo wound healing activity of asioticoside isolated from Centella asiatica. J Ethnopharmacology 1999; 65:1-11. [ Özet ]
16) Bolger WE, Leonard D, Dick EJ, Stierna P. Gram negative sinusitis: A bacteriologic and histologic study in rabbits. Am J Rhinol 1997; 11:15-25. [ Özet ]
17) Culviner WT, Leonard DW, Wilhelmsen CL, Bolger WE. Experimental myospherulosis of the paranasal sinuses. A histologic rabbit study. Am J Rhinol 2000; 14:131-137. [ Özet ]
18) Kakizaki H, Shimada K. Experimental study of the cause of myospherulosis. Am J Clin Pathol 1993; 99:249-256. [ Özet ]




