DIAGNOSTIC IMPORTANCE OF THE RELATIONSHIP AMONG CALORIC TEST EVALUATION PARAMETERS IN PATIENTS WITH UNILATERAL VESTIBULAR WEAKNESS: A CASE-CONTROL STUDY
2Bingöl Üniversitesi, Odyoloji, Bingöl, Türkiye
Summary
Background: To investigate the relationships among caloric test evaluation parameters in individuals with unilateral vestibular weakness.Materials and Methods: The study included 28 individuals in the patient group and 28 individuals in the control group. A bithermal caloric test was administered to all participants using an air irrigator. The measured parameters included slow phase velocity, latency, time to reach the maximum slow phase velocity, and the difference between the time to reach the maximum slow phase velocity of the caloric nystagmus and the latency.
Results: In comparison with the healthy ears of the patient group, the pathological ears exhibited a significant decrease in slow phase velocity under both cold and warm stimuli, a prolonged latency, and a reduced difference between the time to reach maximum slow phase velocity and the latency period (p<0.05). Additionally, when compared with the control group, the pathological ears of the patient group demonstrated a significant reduction in slow phase velocity under cold and warm stimuli, as well as a shorter time to reach maximum slow phase velocity (p<0.05). Furthermore, the healthy ears of the patient group showed an increase in slow phase velocity under cold stimuli and a decrease in latency under both cold and warm stimuli when compared with the control group (p<0.05). Both positive and negative correlations were identified among the caloric test evaluation parameters.
Conclusions: In addition to the maximum slow phase velocity of caloric nystagmus, other evaluation parameters may also contribute to the diagnostic process. Moreover, the relationship between the velocity storage mechanism and the caloric test parameters, which assess the low-frequency VOR, is particularly noteworthy.
Introduction
The caloric test was first described by Barany.[1] It has since become a fundamental method for evaluating the peripheral vestibular system and is widely employed to assess the horizontal vestibulo-ocular reflex (h-VOR) within a frequency range of 0.002-0.004 Hz.[2] The cristae ampullaris, which serve as the receptors for the angular VOR, contain both type I and type II hair cells. Type I hair cells, which are connected to irregular afferent fibers, are located centrally within the cristae and exhibit greater sensitivity to high-frequency stimuli. In contrast, type II hair cells, connected to regular afferent fibers and located peripherally within the cristae, are more responsive to low-frequency stimuli.[3-4] Given that the caloric test assesses low-frequency h-VOR function, type II hair cells are more sensitive to caloric stimulation.[5]The basic mechanism of vestibular stimulation in caloric testing is based on the movement of endolymph induced by temperature variations.[6,7] The test parameters can be analyzed by recording the nystagmus elicited during caloric stimulation using videonystagmography (VNG). The caloric test enables separate evaluation of the lateral semicircular canals on each side and is therefore considered the gold standard for diagnosing unilateral weakness.[6,7] Unilateral weakness refers to hypofunction on the affected side due to impaired VOR function.
Since the initial definition of the caloric test, various parameters-such as the duration, latency, amplitude, frequency, and maximum slow phase velocity of nystagmus-have been evaluated to interpret test results.[6,10-13] While the duration of nystagmus was initially the primary parameter used for interpretation, advancements in measurement techniques enabled by technological progress have made it possible to assess additional quantitative characteristics of nystagmus.[14] Among these, the maximum slow phase velocity is regarded as the fundamental parameter and is considered the most reliable value for interpreting caloric test outcomes.[2,10,15-18] A review of the literature reveals a limited number of studies examining the relationship between maximum slow phase velocity and the duration of nystagmus.[10-13,19,20] Skipper et al. reported a mean total nystagmus duration of 183.9 seconds for cold and warm caloric stimuli. In the same study, no significant relationship was found between maximum slow phase velocity and nystagmus duration.[10] Vesterhauge et al. concluded that maximum slow phase velocity provided more reliable diagnostic information than the time parameter.[19] Maire et al. explored the diagnostic significance of nystagmus frequency and duration in addition to maximum slow phase velocity, reporting a strong correlation among all three parameters of caloric nystagmus.[11] A review of the literature reveals no studies investigating the diagnostic significance of the correlations among the following parameters: the slow phase velocity of caloric nystagmus, latency, time to reach the maximum slow phase velocity, and the difference between the time to reach the maximum slow phase velocity and latency. In this context, the present study offers a novel perspective to the existing literature. The aim of this study was to examine the relationships among caloric test evaluation parameters in individuals with unilateral vestibular weakness.
Methods
Study designThis study employed a case-control research design, as the caloric test evaluation parameters were compared between individuals with unilateral vestibular weakness and healthy controls.
Participants
The research was conducted prospectively in the Audiology Unit of the Otorhinolaryngology Polyclinic at a University Hospital between October 2021 and February 2022. Ethical approval was obtained from the Clinical Research Ethics Committee of a local University Institute of Health Sciences (Decision number: 2021/159), and informed consent was obtained from all participants. A total of 56 individuals (112 ears) were included in the study, with 28 individuals (56 ears) assigned to the patient group and 28 individuals (56 ears) to the control group. Exclusion criteria included being under 18 years of age; having communicative or cognitive impairments; systemic disease; conductive hearing loss; acute vestibular disease; spontaneous nystagmus; or a central vestibular disorder.
Equipment and procedure
The caloric test was conducted using an air irrigator (Air FX, Interacoustics AS, Denmark). Caloric nystagmus induced during the test was recorded using a videonystagmography device (VisualEyes 4-channel, Micromedical Technologies, Chatham, USA).
All individuals included in the study were examined by an otolaryngologist. To assess eligibility based on the inclusion criteria, a comprehensive anamnesis was obtained, and the following tests were administered: pure tone audiometry, immittancemetry, oculomotor tests, spontaneous nystagmus test, positional tests, head shake test, caloric test, video head impulse test, cervical and ocular vestibular tests, and evoked myogenic potentials. Based on the test results, individuals diagnosed with unilateral weakness were assigned to the patient group, while those with normal findings constituted the control group.
The caloric test procedure was conducted using the same protocol for both the patient and control groups. A bithermal binaural air caloric test was performed with participants in the supine position and their heads flexed at a 30° angle. Caloric stimulation was administered through the external auditory canal using air at 24°C for cold irrigation and 50°C for warm irrigation. The sequence of stimulation was as follows: cold air to the left ear, cold air to the right ear, warm air to the left ear, and warm air to the right ear. A 5-minute break was given between interaural stimulations and a 7-minute break between intertemporal stimulations. Each caloric irrigation lasted for 60 seconds, and the resulting caloric nystagmus was recorded for an additional 60 seconds.
Evaluation parameters
- The maximum slow phase velocities of nystagmus following cold and warm caloric stimuli were determined by analyzing the 10-second interval during which the response reached its highest intensity within the 60 seconds following the caloric stimulus.
- The rate of unilateral weakness in the caloric test was calculated automatically by the device using the Jongkees formula.
- A unilateral weakness greater than 20% was considered pathological.
- The onset of nystagmus was defined as the point at which the slow phase velocity exceeded 3°/sec.
- The latent period of nystagmus was defined as the time from the application of the caloric stimulus to the point at which the slow phase velocity reached 3°/sec.
- The time to reach maximum slow phase velocity was defined as the duration from the application of the caloric stimulus to the point at which the maximum velocity occurred.
- The difference between the time to reach maximum slow phase velocity and the latent period represented the duration required for the nystagmus to reach its peak slow phase velocity following the latent period.
- A graphical representation of the caloric test evaluation parameters is shown in Figure 1.
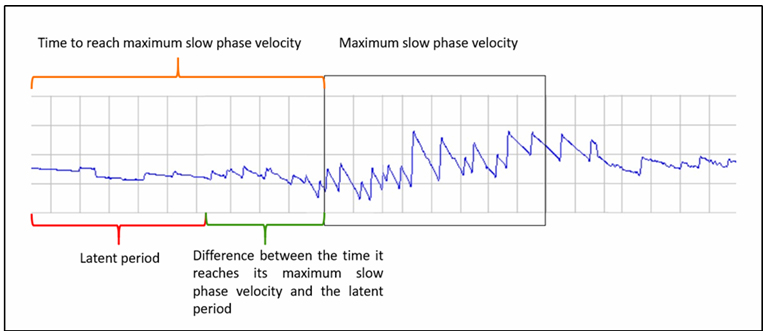 Büyütmek İçin Tıklayın |
Figure 1: Graphical representation of caloric test evaluation parameters. |
Statistical analysis
Data analysis was performed using the SPSS (Statistical Program for Social Sciences) version 25. The Kolmogorov-Smirnov test was employed to assess whether the data followed a normal distribution.[21] A significance level of p = 0.05 was adopted for all comparative analyses. As the variables did not exhibit a normal distribution (p > 0.05), non-parametric statistical methods were used. Comparisons between independent groups were conducted using the Mann-Whitney U test due to the violation of the normality assumption. Spearman rank correlation coefficient was used to test the relationship between variables. For the analysis of categorical data, cross-tabulations were created and Chi-square (χ²) analysis was applied. The Spearman rank correlation coefficient was used to evaluate the direction and strength of relationships between variables.
Results
Among the participants included in the study, the patient group consisted of 10 individuals (35.70%) diagnosed with vestibular neuritis and 18 individuals (64.30%) diagnosed with Meniere's disease. The groups demonstrated a homogeneous distribution in terms of age and side of weakness. A statistically significant difference was observed between the patient and control groups in the percentage of unilateral weakness (p < 0.05, Table 1).Table 1: Comparison of Groups by Demographic Variables
Intra-Group Comparisons in the Patient Group
A statistically significant difference was observed between the pathological and healthy ears of the patient group in terms of slow phase velocity, latency, and the difference between the time to reach maximum slow phase velocity and the latency of nystagmus under both cold and warm stimuli (p < 0.05, Table 2).
Comparisons Between Groups
A statistically significant difference was found between the pathological ears of the patient group and the ears of the control group in terms of the slow phase velocity of nystagmus under cold stimulus (p < 0.05). Under warm stimulus, a statistically significant difference was observed between the pathological ears of the patient group and the control group ears in terms of slow phase velocity, time to reach maximum slow phase velocity, and latency (p < 0.05, Table 3).
A statistically significant difference was observed between the healthy ears of the patient group and the ears of the control group in terms of slow phase velocity and latency parameters of nystagmus under cold stimulus (p < 0.05). Under warm stimulus, a statistically significant difference was found between the healthy ears of the patient group and the control group ears in terms of the latency parameter (p < 0.05, Table 4).
A correlation was identified between the parameters obtained from the pathological ears and those from the healthy ears of the patient group under cold air stimulation. Specifically, a statistically significant, positive, and moderate correlation was found between the slow phase velocities of the two ears; between the times to reach maximum slow phase velocity; and between the slow phase velocity and both the maximum intensity duration and the difference between the time to reach maximum slow phase velocity and the latency (p < 0.05). Under warm air stimulation, a statistically significant moderate positive correlation was observed between the time to reach maximum slow phase velocity in the pathological ears and the maximum intensity time in the healthy ears of the case group (p < 0.05, Table 5).
Table 5: Correlations between caloric test evaluation parameters in warm and cold air stimulus.
Discussion
The caloric test induces vestibular stimulation through thermal stimuli, independent of angular head movements. It allows for the independent evaluation of labyrinthine function on both sides, making it a valuable diagnostic tool for identifying unilateral weakness.[6,8,9] Among the quantitative parameters of the caloric test, the maximum slow phase velocity of nystagmus is regarded as the primary evaluation metric.[10,15] However, based on the findings of the present study, it is suggested that in individuals with unilateral weakness, parameters such as latency, time to reach maximum slow phase velocity, and the difference between the time to reach maximum slow phase velocity and latency-alongside the maximum slow phase velocity of nystagmus-may also contribute to the diagnostic process.The results of this study may be associated with the velocity storage mechanism, which is known to be impaired in the presence of unilateral weakness.[22] The velocity storage mechanism involves a central process that evaluates the accuracy of information transmitted by the vestibular system.[23-25] This process is mediated through the prolongation of vestibular signals, which can be observed in the vestibular nuclei.[26] It has been reported that the velocity storage mechanism particularly extends the duration of low-frequency vestibular responses.[27-29] Loss or partial impairment of the velocity storage mechanism due to unilateral weakness may lead to a reduction in the overall duration of the vestibular response. These pathophysiological changes may account for the prolonged latency of nystagmus observed in the pathological ears of individuals with unilateral weakness in the present study, as well as the corresponding decrease in the difference between the time at which nystagmus reaches its maximum slow phase velocity and the latency period. In the present study, the slow phase velocity, latency, and the difference between the time at which nystagmus reached maximum slow phase velocity and the latency period were statistically significantly reduced in the pathological ears of the patient group compared to their healthy ears, primarily due to the delayed latency. Based on this finding, it is suggested that the latent period may hold diagnostic significance. Wodak also reported that the latent period could be a valuable parameter in the diagnostic process.[30] Based on the findings, it is believed that the delayed latent period is attributable to the same underlying mechanism. However, other authors have suggested that the latent period is influenced more by blood flow than by the functional status of the vestibular apparatus.[31,32] No comparable study was identified in the literature in which the specific parameters evaluated in the present research were directly compared. Only a limited number of studies have assessed caloric test parameters from different perspectives. In a study by Wade et al., the duration of nystagmus recorded in the pathological ears of individuals with unilateral weakness was found to decrease proportionally with increasing canal paresis, and this reduction was attributed to the horizontal slow phase velocity storage mechanism.[33] Krstulovic et al. examined individuals with Meniere"s disease and suggested that the velocity storage mechanism is enhanced in individuals with labyrinthine disorders.[34] The negative effects observed in the evaluated parameters following caloric stimulation in the present study support the theory that the velocity storage mechanism specifically prolongs the duration of low-frequency vestibular responses. However, further experimental studies are needed to explore the physiological relationship between unilateral weakness and the velocity storage mechanism.
In the present study, the slow phase velocity of nystagmus elicited by cold air caloric stimulation was significantly lower in the ears of the control group compared to the healthy ears of the patient group. Additionally, the latency of nystagmus under both cold and warm air caloric stimulation was significantly longer in the control group than in the healthy ears of the patient group. The results obtained in the present study using an air irrigator may be influenced by the inherent limitations of this irrigation method. Air caloric irrigators are more susceptible to environmental conditions compared to water-based systems. While water enables more efficient transmission of thermal energy to the inner ear, air is more rapidly affected by ambient temperature, which may compromise the delivery of thermal energy at the intended temperature.[2] This limitation may help explain the observed differences between the healthy ears of the case group and the ears of the control group. Additionally, individual anatomical variations in the external auditory canal may contribute to differences in caloric stimulation between the right and left ears. Proctor and Glackin reported that caloric stimulation tends to be more intense in individuals with narrower external auditory canals.[35] However, as quantitative measurements of the external auditory canal were not conducted in the present study, this represents a limitation. Future research is recommended to investigate the influence of structural characteristics and individual variations of the tested ear on caloric test outcomes.
This study highlights the diagnostic value of the correlations among caloric test evaluation parameters. In pathological ears with reduced maximum slow phase velocity, a delayed latency and prolonged time to reach maximum slow phase velocity were observed. Conversely, in healthy ears with higher maximum slow phase velocity, both the latency and the time to reach maximum slow phase velocity were shorter. No studies in the literature have examined the correlations among these specific parameters. In this regard, the present study provides a novel perspective. However, only a limited number of studies have investigated the correlation between the maximum slow phase velocity of nystagmus and its total duration. Skipper et al.[10], Mulch et al.[12], Vesterhauge and Larsen[13], Vesterhauge et al.[19], and Karlsen et al.[20] reported no significant correlation between the maximum slow phase velocity of nystagmus and its total duration. These studies emphasized that the maximum slow phase velocity is the most reliable parameter for diagnostic purposes. Additionally, it has been suggested that the total duration of caloric nystagmus is related to the physical response induced by the thermal stimulus, while the maximum slow phase velocity and duration parameters of nystagmus reflect distinct functions of vestibular activity and assess different aspects of the VOR.[12,36] Maire et al. reported a strong correlation among maximum slow phase velocity, total duration, and frequency of nystagmus.[11] Similarly, the findings of the present study revealed a moderate positive correlation between the maximum slow phase velocity of nystagmus and the difference between the time to reach maximum slow phase velocity and the latency period. The observed correlation between maximum slow phase velocity and latency-related parameters highlights the potential diagnostic value of incorporating these measures into the evaluation process.
The primary limitation of this study was the limited number of relevant studies in the literature that could be used for comparison. Additionally, the evaluations were conducted based on unilateral weakness resulting from vestibular disorders, rather than specific vestibular disorder classifications. This limitation was attributed to the small sample size within individual vestibular disorder categories. A larger sample size may enable assessments to be conducted according to specific vestibular disorder classifications and allow for the generation of more independent data. It has been reported that the structure of the external auditory canal influences the outcomes of caloric stimulation. The absence of quantitative measurements of the external auditory canal was identified as a limitation of the present study. Another limitation is the use of an air caloric irrigator instead of a water caloric irrigator. Given that the air caloric irrigator is more susceptible to environmental conditions, it is recommended that future studies employ a water caloric irrigator for improved reliability.
Conclusion
The relationship between the maximum slow phase velocity of nystagmus recorded during the caloric test and other associated parameters is noteworthy. The findings of this study may provide preliminary evidence that, in addition to the maximum slow phase velocity, parameters such as latency, time to reach maximum slow phase velocity, and the difference between the time to reach maximum slow phase velocity and latency may also contribute to the diagnostic process in individuals with unilateral weakness. Additionally, a thorough understanding of the underlying physiological mechanisms associated with the latency parameters of caloric nystagmus is essential for accurately interpreting the relationships among these measures. Given that caloric stimulation involves low-frequency input, these parameters are likely to influence distinct pathways of the VOR and may be associated with the velocity storage system. This study is considered one of the first in the literature to examine the relationships and correlations among caloric test parameters collectively. Future research with larger sample sizes may yield more robust data for elucidating the pathophysiological relationships among these parameters.Informed Consent: Informed consent was obtained from all individual participants included in the study.
Competing Interests: The authors have no conflict of interest to declare.
Funding: The authors did not receive support from any organization for the submitted work. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgements: We would like to thanks, our patients who participated in our study for their contribution to the studies.
Reference
1) Barany R. Untersuchengen uber den vom vestibulapparat des ohres, seinen reflektorisch ausgelosten thytmischen nystagmus und seine begleiterscheinungen. Monatsschr Ohren. 1906;40:193-297.
2) Shepard NT, Jacobson GP. The caloric irrigation test. Handb Clin Neurol. 2016;137:119-31. doi: 10.1016/B978-0-444-63437-5.00009-1. [ Özet ]
3) Mezzalira R, Bittar RSM, do Carmo Bilécki-Stipsky MM, Brugnera C, Grasel SS. Sensitivity of caloric test and video head impulse as screening test for chronic vestibular complaints. Clinics (Sao Paulo). 2017;72(8):469-473. doi: 10.6061/clinics/2017(08)03. [ Özet ]
4) McCaslin DL, Rivas A, Jacobson GP, Bennett ML. The dissociation of video head impulse test (vHIT) and bithermal caloric test results provide topological localization of vestibular system impairment in patients with "definite" Ménière's disease. Am J Audiol. 2015;24(1):1-10. doi: 10.1044/2014_AJA-14-0040. [ Özet ]
5) Hamann KF. Vibration-Induced Nystagmus: A Biomarker for Vestibular Deficits - A Synopsis. ORL J Otorhinolaryngol Relat Spec. 2017;79(1-2):112-120. doi: 10.1159/000455720. [ Özet ]
6) Aghababaei Ziarati M, Taziki MH, Hosseini SM. Autonomic laterality in caloric vestibular stimulation. World J Cardiol. 2020;12(4):144-154. doi: 10.4330/wjc.v12.i4.144. [ Özet ]
7) Molnár A, Maihoub S, Tamás L, Szirmai Á. Comparison between caloric and video-head impulse tests in Ménière's disease and vestibular neuritis. Int J Audiol. 2022;19:1-7. doi: 10.1080/14992027.2022.2059711. [ Özet ]
8) Lee SU, Kim HJ, Choi JY, Koo JW, Kim JS. Evolution of caloric responses during and between the attacks of Meniere's disease. J Neurol. 2021;268(8):2913-2921. doi: 10.1007/s00415-021-10470-4. [ Özet ]
9) Cunha LC, Felipe L, Carvalho SA, Labanca L, Tavares MC, Gonçalves DU. Validity of the monothermal caloric testing when compared to bithermal stimulation. Pro Fono. 2010;22(1):67-70. doi: 10.1590/s0104-56872010000100013. [ Özet ]
10) Skipper C, Knight R, Cane D. Nystagmus duration after caloric irrigations. Int J Audiol. 2020;59(5):333-340. doi: 10.1080/14992027.2019.1703046. [ Özet ]
11) Maire R, Daoui B, Van Melle G. Evaluation of the caloric test by combining 3 response parameters. Otolaryngol Head Neck Surg. 2000;122(6):814-20. doi: 10.1016/S0194-59980070007-6. [ Özet ]
12) Mulch G, Leonardy B, Petermann W. Which are the parameters of choice from the evaluation of caloric nystagmus? Arch Otorhinolaryngol. 1978;221(1):23-35. doi: 10.1007/BF00456381. [ Özet ]
13) Vesterhauge S, Kildegaard Larsen P. Normal values in a routine ENG test. Acta Otolaryngol. 1977;84(1-2):91-7. doi: 10.3109/00016487709123946. [ Özet ]
14) Furman JM, Jacob RG. Jongkees' formula re-evaluated: order effects in the response to alternate binaural bithermal caloric stimulation using closed-loop irrigation. Acta Otolaryngol. 1993;113(1):3-10. doi: 10.3109/00016489309135759. [ Özet ]
15) Starkov D, Strupp M, Pleshkov M, Kingma H, van de Berg R. Diagnosing vestibular hypofunction: an update. J Neurol. 2021;268(1):377-385. doi: 10.1007/s00415-020-10139-4. [ Özet ]
16) Cerchiai N, Navari E, Miccoli M, Casani AP. Menière's Disease and Caloric Stimulation: Some News from an Old Test. J Int Adv Otol. 2019;15(3):442-446. doi: 10.5152/iao.2019.7430. [ Özet ]
17) Adams ME, Telian SA, Kane RL, Butler M. Monothermal Caloric Screening Test Accuracy: A Systematic Review. Otolaryngol Head Neck Surg. 2016;154(6):982-96. doi: 10.1177/0194599816630963. [ Özet ]
18) Thatcher AL, Beckerman ML, Telian SA, King WM. Monothermal Caloric Screening to Improve Healthcare Value. Ear Hear. 2016;37(3):e188-93. doi: 10.1097/AUD.0000000000000262. [ Özet ]
19) Vesterhauge S, Holm-Jensen S, Osterhammel D, Peitersen E. Caloric testing with small temperature gradients. Caloric zero. ORL J Otorhinolaryngol Relat Spec. 1984;46(2):105-10. doi: 10.1159/000275694. [ Özet ]
20) Karlsen EA, Hassanein RM, Goetzinger CP. The effects of age, sex, hearing loss and water temperature on caloric nystagmus. Laryngoscope. 1981;91(4):620-7. doi: 10.1288/00005537-198104000-00017. [ Özet ]
21) Alpar R. Spor, Sağlık ve Eğitim Bilimlerinde Örneklerle Uygulamalı İstatistik ve Geçerlik-Güvenirlik. 6. Baskı. Ankara: Detay Yayıncılık, 2020.
22) Hain TC, Zee DS. Velocity storage in labyrinthine disorders. Ann N Y Acad Sci. 1992;656:297-304. doi: 10.1111/j.1749-6632.1992.tb25216.x. [ Özet ]
23) Raphan T, Cohen B. Velocity storage and the ocular response to multidimensional vestibular stimuli. Rev Oculomot Res. 1985;1:123-43. [ Özet ]
24) Hess BJ, Angelaki DE. Inertial vestibular coding of motion: concepts and evidence. Curr Opin Neurobiol. 1997;7(6):860-6. doi: 10.1016/s0959-4388(97)80147-x.
25) MacNeilage PR, Ganesan N, Angelaki DE. Computational approaches to spatial orientation: from transfer functions to dynamic Bayesian inference. J Neurophysiol. 2008;100(6):2981-96. doi: 10.1152/jn.90677.2008. [ Özet ]
26) Dai M, Klein A, Cohen B, Raphan T. Model-based study of the human cupular time constant. J Vestib Res. 1999;9(4):293-301. [ Özet ]
27) Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res. 1992;90(3):526-38. doi: 10.1007/BF00230935. [ Özet ]
28) Demer JL, Robinson DA. Different time constants for optokinetic and vestibular nystagmus with a single velocity-storage element. Brain Res. 1983;276(1):173-7. doi: 10.1016/0006-8993(83)90560-7. [ Özet ]
29) Jacobson GP, McCaslin DL, Patel S, Barin K, Ramadan NM. Functional and anatomical correlates of impaired velocity storage. J Am Acad Audiol. 2004;15(4):324-33. doi: 10.3766/jaaa.15.4.6. [ Özet ]
30) Wodak E. Dıagnostıc value of the latency perıod after calorıc stımulatıon of vestıbular apparatus. AMA Arch Otolaryngol, 1952; 55(3):381-386. [ Özet ]
31) Schmaltz G.The physical phenomena occurring in the semicircular canals during rotatory and thermic stimulation. Proc Roy Soc Med. 1932;25: 359-381.
32) Dohlman G. On the latency time of caloric nystagmus. Acta oto-laryng. (Supp.) 1925;5:1.
33) Wade SW, Halmagyi GM, Black FO, McGarvie LA. Time constant of nystagmus slow-phase velocity to yaw-axis rotation as a function of the severity of unilateral caloric paresis. Am J Otol. 2000;20(4):471-8. [ Özet ]
34) Krstulovic C, Atrache Al Attrache N, Pérez Garrigues H, Argente-Escrig H, Bataller Alberola L, Morera Pérez C. Increased Velocity Storage in Subjects with Meniere's Disease. J Int Adv Otol. 2016;12(1):87-91. doi: 10.5152/iao.2016.1947. [ Özet ]
35) Proctor L, Glackin R. Factors contributing to variability of caloric test scores. Acta Otolaryngol. 1985;100(3-4):161-71. doi: 10.3109/00016488509104778. [ Özet ]
36) Luxon L. Comparison of assessment of caloric nystagmus by observation of duration and by electronystagmographic measurement of slow-phase velocity. Br J Audiol. 1995;29(2):107-15. doi: 10.3109/03005369509086587. [ Özet ]




