CIGARETTE SMOKING EFFECT ON EFFERENT AUDITORY SYSTEM: A CASE CONTROL STUDY
2Marmara Üniversitesi Tıp Fakültesi, Odyoloji, İstanbul, Turkey
Summary
Purpose: Smoking is known to cause ototoxicity, hypoxia in cochlear and spiral gaglion cells and an increase in blood viscosity. It was also found that nicotine binds to nicotinic acetylcholine receptors and eliminates the modulatory effects of the receptors. Considering that cholinergic neurons in the upper auditory pathways are also located in the olivo-cochlear pathway, our study aimed to evaluate the peripheral and efferent auditory systems in smokers.Methods: In the study, TEOAE and contralateral suppression of TEOAE responses were evaluated in 20 smoker aged 18-30 who had smoked for at least 2 years and 20 non-smokers aged 18-30.
Results: TEOAE-2000Hz, TEOAE-2800 Hz and TEOAE-4000 Hz values were significantly decreased in the group of smokers. There was no significant difference in the suppressed emission values of the ears and in the numbers of suppressed ears between the groups.
Conclusions: Although the findings obtained in our study show that smoking may cause cochlear effects, no findings were obtained indicating that smoking affects the medial olivocochlear (MOC) reflex. This situation is thought to be related to the fact that the study was conducted on young individuals with a short smoking duration. In future studies, it is recommended to use additional tests to reveal the efferent auditory system effects.
Introduction
Hearing loss is one of the most common sensory disorders that interferes with the ability to understand speech, causing social and communication problems[1]. There are various risk factors for hearing loss, such as genetic factors, congenital complications, infectious diseases, ototoxic drug use, noise exposure, and old age[2]. In addition, smoking, which is a serious public health problem; It is known to cause auditory problems in addition to bacterial respiratory infections, acute and chronic viral diseases, mouth, larynx, esophagus, pancreas, kidney and bladder cancer, arteriosclerosis, aortic aneurysm, stroke and multiple organ disorders[3-6].Studies have reported that smoking is a risk factor for sensorineural hearing loss, and this has been associated with the ototoxic effects of various components such as toluene, benzene and carbon monoxide found in cigarette smoke[3,7,8]. Hawkins (1971) observed nicotine receptors in the hair cells of the subjects after cigarette exposure[9]. Although the mechanism of action of smoking has not been clearly determined, many studies have drawn attention to cochlear ischemia due to increased carboxyhemoglobin levels, and the increase in blood viscosity caused by smoking, in addition to the ototoxicity of nicotine[6,10,11]. Smoking has also been reported to reduce oxygen levels in spiral ganglion cells.
Furthermore, studies have shown that nicotine binds to nicotinic acetylcholine receptors (nAChR), which modulate the effects of acetylcholine and extend from the trapezoid body to the cochlear nucleus, abolishing the modulatory effects of the receptors and causing central impairments in auditory and visual modalities[12-15]. Cholinergic neurons are nerve cells that use acetylcholine (ACh) as a neurotransmitter[16], the portion of these neurons in the upper auditory pathways has been reported to involve the olivocochlear pathway[17]. In the light of these data, we suggest that smoking may affect cochlea and medial olivocochlear (MOC) reflex function. The olivocochlear bundle plays an inhibitory role on the activity of outer hair cells and its stimulation decreases auditory nerve response, basilar membrane motility and OAE amplitüde. Contralateral suppressed OAE is the only objective and non-invasive method to assess MOC activity[18]. Vinay et al. (2010) observed a decrease in the value of suppression in smokers aged 20-69, while Paschoal et al. (2009) observed an increase. The number of studies on this subject in the literature is insufficient and conflicting results have been reported in current studies.[19,20]. Therefore, in our study, we aimed to evaluate the cochlear and contralateral efferent suppression effect due to smoking by performing TEOAE test in the presence and absence of contralateral stimuli.
Methods
This study was conducted in Bezmialem Vakif University, Audiology Department and approval was obtained from Bezmialem Vakif University Ethics Committee. Number of the ethics committee: 2021/409.
1 Participants
Twenty smokers and 20 non-smokers between the ages of 18-30 were included in our study. The sample size for %80 power and at %95 confidence level and 0.05 significance level was determined as at least 15 as a result of power analysis. People who have been smoking for at least 2 years were included in the smokers group. As the participants in the smokers group were young adult, their maximum duration of smoking was 5 years. The amount of cigarette use of the participants was calculated as pack/year. The pack/year calculation was obtained by dividing the number of cigarettes used per day by 20 and multiplying by the year of use[19]. Our participants smoked a minimum of 1 pack year and a maximum of 2.5 pack years. The non-smoking group consisted of individuals with no smoking history and normal hearing. To verify bilateral normal middle ear function, all participants underwent immitansmetric evaluation at 226 Hz and acoustic reflex testing at 500-1000-2000-4000 Hz with GSI's Tympstar Pro device. In order to evaluate the auditory sensitivity of the patients, 125-8000 Hz air conduction thresholds and 250-4000 Hz bone conduction thresholds were evaluated with the Madsen Astera device. Individuals with pure tone average of air and bone conduction better than 20 dB HL at frequencies of 500-1000-2000-4000 Hz, bilateral Type-A tympanograms, and ipsilateral/contralateral acoustic reflexes at frequencies of 500, 1000, 2000, and 4000 Hz were included in the non-smoker group.
All participants did not have auditory-vestibular complaints, history of noise exposure, head trauma, ototoxic drug use, otological, central, systemic and metabolic diseases. Verbal and written consent were obtained from each participant of the study.
2 Procedures
2.1 Transient Evoked Otoacoustic Emissions Test
Transient evoked otoacoustic emissions (TEOAE) measurement was recorded with linear click stimulus at 75±4 dB peSPL at frequencies of 1000, 1400, 2000, 2800 and 4000 Hz with ILO 292 Echoport USB II device. Reproducibility [70% and above], stability [80% and above], stimulus intensity [75± 4 dB peSPL and SNR>3 dB] parameters were followed.
2.2 Contralateral Suppression of Transient Evoked Otoacoustic Emissions
ILO 292 Echoport USB II device was used for contralateral suppression test with otoacoustic emissions. Separately for right and left ears, it was recorded with linear click stimulus at 75± 4 dB peSPL in the presence of broadband white noise provided with contralateral 2 sec intervals with 60 dB SPL intensity at frequencies of 1000, 1400, 2000, 2800 and 4000 Hz. The followed parameters were as follows: reproducibility [70% and above], stability [80% and above], stimulus intensity [75± 4 dB peSPL], contralateral stimulus intensity [60 dB SPL] sweep [260] and broadband white noise as contralateral noise type. Presence of suppression was decided if there was at least 1 dB amplitude decrease in at least 3 frequencies.
Statistical Analysis
The results were analyzed using IBM SPSS Statistics version 22.0 software. For the analyzed data, mean, standard deviation, minimum, and maximum values were obtained. The normality analysis of the distribution of continuous numerical values was performed with the Shapiro-Wilk and Kolmogorov-Smirnov tests. A comparison between two variables was performed using the t-test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. The results of all analyses were interpreted at a 95% confidence interval and a significance level of p < 0.05.
Results
The mean ages of the smoking group (15female/5male) and non-smoking group (18 female/2 male) were 22±1.41 and 22.5±0.70, respectively. No statistically difference was found between the groups according to age (p>0,05)In audiometric evaluation of the participants, 125, 250, 4000 Hz air conduction thresholds and 250, 2000, 4000 Hz bone conduction thresholds were lower in the smokers' groups (Table-1).
Table 1: Audiometric thresholds of groups
TEOAE-1000, TEOAE-1400, TEOAE-2000, TEOAE-2800, TEOAE-4000 values were compared in order to evaluate cochlear sensitivity between groups with Mann-Whitney U test. TEOAE-2000Hz, TEOAE-2800 Hz and TEOAE-4000 Hz amplitude values were lower in the smokers' group (Figure-1).
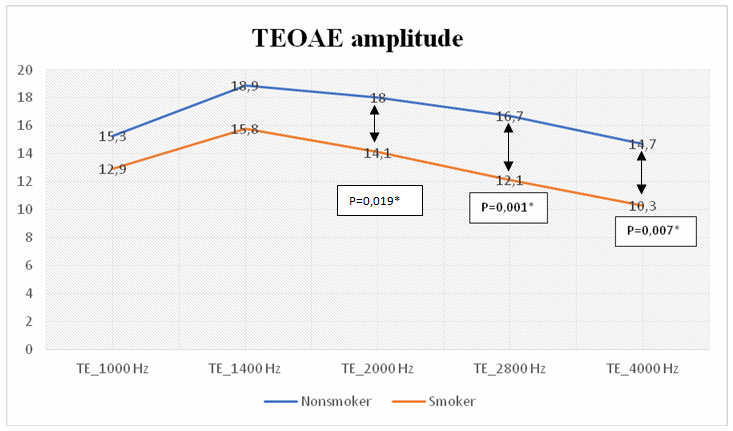 Büyütmek İçin Tıklayın |
Figure 1: TEOAE amplitude value of groups |
The suppression values at 1000 Hz, 1400 Hz, 2000 Hz, 2800 Hz, and 4000 Hz in the right and left ears of participants in the smokers' and non-smokers' groups were compared using the t-test. There was no significant difference in the emission values of the right and left ears in both groups. Since there was no significant difference between the ears in both groups, the total ear (n:40/40) was taken as the basis for the comparison of suppressed emission values between the groups. Suppression was obtained in 12 of 40 ears in the non-smoking group, while suppression was obtained in 13 of 40 ears in the smoking group. No significant difference was obtained in the number of suppressed ears between the groups (p=0,80; p>0,05). The suppressed emission values between the groups were compared with the t test, and there was no significant difference in the suppressed emission values of the ears in the smoking and non-smoking groups (p>0,05) (Table-2).
Table 2: Suppressed emission values of the ears in the smoking and non-smoking groups
Discussion
It is stated in the literature that smoking affects the hearing system. The risk of hearing loss was found to be 1.69 times higher in smokers[21]. In studies, increased hearing thresholds have been observed in smokers, especially in the high frequency region[5]. In our study, although the hearing thresholds of both groups were within normal limits, a significant decrease in 125, 250 and 4000 Hz air conduction thresholds was observed in the smoker group. In the longer term, it is thought that this decrease will be more pronounced with advancing age, metabolic diseases and noise exposure[19,22]. Similarly, in OAE studies conducted in smokers, although the amplitude decreases are generally in the high frequency region such as 4000 Hz and 6000 Hz, there are also studies in which an amplitude decrease is observed at 1000 Hz[20,23]. This influence in the high frequency region is explained by the fact that the cochlear artery feeding the basal region of the cochlea is more sensitive to atherosclerotic changes seen in smokers. TE2000 Hz, TE2800 Hz and TE4000 Hz values were significantly lower in the smoker group in our study, also. Some studies in the literature have reported that smoking alone cannot cause auditory effects, but may increase the effect of noise induced hearing loss. People who were exposed to noise and had diseases such as hypertension, diabetes and high cholesterol that could make the stria vascularis vulnerable to vascular danger were not included in our study. Despite this situation, the decrease in amplitude observed in TEOAE in the smoking group shows that smoking alone may cause cochlear damage.Ryan (1990) showed in their animal experiment that the central effects of nicotine can modulate the response of hair cells through efferent neural discharge changes in the olivo-cochlear bundle pathway[24]. Vinay (2010), in his study investigating the age-related suppression levels in smokers, found that suppression values decreased in all age groups in smokers. In addition, suppression values in individuals aged 20-49 were higher than in individuals aged 50-69 in the smoker group[19]. In contrast, Paschoal et al. (2009) revealed in their study that smoking increases suppression. They found that this result was associated with an increase in inhibition due to the stimulatory effect of nicotine on the acetylcholine that efferent auditory neurotransmitter. In our study, no significant difference was found in the suppression values and the number of suppressed ears between the smoker and non-smoker groups. We think that these results may be related to the short duration of smoking due to the fact that our study sample consists of young adults between the ages of 18-30. Considering the studies, it is recommended to conduct efferent system studies in elderly individuals with long-term smoking. In addition, we think that the contradictory findings in our study with Vinay's (2010) study may be related to the duration of smoking, passive smoking, smoking dose, nicotine and carbon monoxide ratios of the cigarette used.
Conclusion
Although the findings obtained in our study show that smoking may cause cochlear effects, no findings were obtained indicating that smoking affects the medial olivocochlear (MOC) reflex. This situation is thought to be related to the fact that the study was conducted on young individuals with a short smoking duration. In future studies, it is recommended to use additional tests to reveal the efferent auditory system effects.
Competing Interests
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from public, commercial, or not- for-profit funding agencies.
Authors' contributions
MBB: Hypothesis creation, study planning, literature review, data collection, statistical analysis, writing of the article
NTE: literature review, writing of the article
AP: data collection
AZP: data collection
Bİ: data collection
NB: statistical analysis, writing of the article
ÖGT: writing of the article
Reference
1) Martínez-Pérez B, De La Torre-Díez I, López-Coronado M. Mobile health applications for the most prevalent conditions by the World Health Organization: review and analysis. J Med Internet Res. 2013;15(6):e120.
2) Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med. 2008;168(14):1522-30.
3) Sumit AF, Das A, Sharmin Z, Ahsan N, Ohgami N, Kato M, et al. Cigarette smoking causes hearing impairment among Bangladeshi population. PLoS One. 2015;10(3):e0118960. [ Özet ]
4) Rosemberg J. A saúde do fumante. Em: Rosemberg J Tabagismo e Saúde: informação para profissionais de saúde São Paulo: Ministério da saúde. 1987;20-3.
5) Rogha M, Hashemi M, Askari N, Abtahi SH, Sepehrnejad M, Nilforoush MH. Cigarette smoking effect on human cochlea responses. Adv Biomed Res. 2015;4. [ Özet ]
6) Shimada S, Hasegawa K, Wada H, Terashima S, Satoh-Asahara N, Yamakage H, et al. High blood viscosity is closely associated with cigarette smoking and markedly reduced by smoking cessation. Circulation Journal. 2011;75(1):185-9.
7) Noorhassim I, Rampal KG. Multiplicative effect of smoking and age on hearing impairment. Am J Otolaryngol. 1998;19(4):240-3.
8) Siegelaub AB, Friedman GD, Adour K, Seltzer CC. Hearing loss in adults: relation to age, sex, exposure to loud noise, and cigarette smoking. Archives of Environmental Health: An International Journal. 1974;29(2):107-9.
9) Hawkins Jr JE. The role of vasoconstriction in noise-induced hearing loss. Annals of Otology, Rhinology & Laryngology. 1971;80(6):903-13.
10) de Oliveira DCCM. Low and high frequency tonal threshold audiometry: comparing hearing thresholds between smokers and non-smokers. Braz J Otorhinolaryngol. 2009;75(5):738-44. [ Özet ]
11) Unverdorben M, Von Holt K, Winkelmann BR. Smoking and atherosclerotic cardiovascular disease: part II: role of cigarette smoking in cardiovascular disease development. Biomark Med. 2009;3(5):617?53.
12) Morley BJ. Nicotinic cholinergic intercellular communication: implications for the developing auditory system. Hear Res. 2005;206(1-2):74-88. [ Özet ]
13) Lustig LR. Nicotinic acetylcholine receptor structure and function in the efferent auditory system. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology: An Official Publication of the American Association of Anatomists. 2006;288(4):424-34.
14) Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32(12):2453-64.
15) Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. Journal of Neuroscience. 2007;27(49):13491-8. [ Özet ]
16) Hut RA, Van der Zee EA. The cholinergic system, circadian rhythmicity, and time memory. Behavioural brain research. 2011;221(2):466-80.
17) Godfrey DA, Park JL, Ross CD. Choline acetyltransferase and acetylcholinesterase in centrifugal labyrinthine bundles of rats. Hear Res. 1984;14(1):93-106.
18) Ciuman RR. The efferent system or olivocochlear function bundle-fine regulator and protector of hearing perception. Int J Biomed Sci. 2010;6(4):276. [ Özet ]
19) Vinay. Effect of smoking on transient evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx. 2010;37(3):299-302.
20) Paschoal CP, de Azevedo MF. Cigarette smoking as a risk factor for auditory problems. Braz J Otorhinolaryngol. 2009;75(6):893-902.
21) Demir E, Celiker M, Afacan NN, Aydogan E, Balaban GA, Erdivanli OC, et al. Effects of smoking on the auditory system: is there a gender difference? Ear Nose Throat J. 2021;100(3):NP147-51.
22) Sung JH, Sim CS, Lee CR, Yoo CI, Lee H, Kim Y, et al. Relationship of cigarette smoking and hearing loss in workers exposed to occupational noise. Ann Occup Environ Med. 2013;25:1-10.




