EFFECT OF PROPOLIS ON NASAL MUCOSA OF RADIOTHERAPHY APPLIED RATS
2Gaziosmanpasa University Faculty of Dentistry, Department of Oral and Maxillofacial Surgery, Tokat, Turkey
3Sakarya University Faculty of Dentistry, Department of Oral and Maxillofacial Surgery, Sakarya, Turkey
Summary
Objective: The aim of this study is to examine the effect of propolis on radiotherapy (RT) applied rat nasal mucosa.Material and Method: Seven of the rats constituted the control group while 30 were divided into three experimental groups, each with 10 rats. A single dose RT of 15 Gray (Gy) was applied to the head and neck area of the rats in G1, G2 and G3 groups. Following RT, water-soluble propolis was given intraperitoneally every day for two weeks as 100 mg/kg to G2 group and as 200 mg/kg to G3 group.
Results: Histological analyses showed that inflammation score was lowest in the control group and highest in G1 group which received RT only. In the G3 group, the inflammation score decreased to a level similar to that of the control and was statistically different from those of the G1 and G2 groups (p < 0.05). Olfactory epithelial thickness was significantly less in G1 group than in control and G3 groups (p < 0.05). Submucosa thickness of the concha region was significantly different in G1 group (p < 0.05) compared to all other groups, and G2, G3 and control groups had decreasing submucosa thickness values.
Conclusion: Our study is the first to examine the effect of propolis on the nasal mucosa of radiation-exposed rats. Systemic propolis administration was shown to reduce inflammation and to enhance healing of nasal mucosa in radiation-exposed rats in a dose dependent manner.
Introduction
The incidence of head and neck cancers in the world exceeds half a million every year.[1] In Europe alone, 139,000 people are annually diagnosed with head and neck cancers.[2] Radiotherapy (RT) is an important healing method for the treatment of head and neck cancers such as nasopharyngeal cancers and paranasal sinus tumors. However, RT significantly reduces the quality of life due to complications such as mucositis, xerostomia, taste disorders, dysphagia, skin damage and sinusitis.[3]
Radiation-induced nasal mucositis is an inevitable complication in patients receiving RT.[4] Patients with nasopharynx carcinoma have high rates of rhinorrhea, nasal congestion and mucosal hyperemia. This condition is an important source of discomfort for the patient and may even lead to discontinuation of treatment for mucosal healing.[5,
The deterioration of the balance among oxidative stress, oxidants and antioxidants was suggested to play an important role in the pathogenesis of oral and nasal mucositis induced by radiation therapy. Control of oxidative stress development is very important in the prevention and treatment of mucositis. For this purpose, antioxidant agents are considered to be useful.[
Methods
Experimental AnimalsAll applications within the scope of the study were carried out with the permission of the Local Ethics Committee for Animal Experiments (Approval No: 2019-HADYEK-37) and in accordance with the ethics laws of the Helsinki Declaration regarding Animal Experiments. In the study, 37 young-adult male Wistar-Albino rats weighing between 250 and 300 g were used. Rats were kept at a constant temperature (21 ± 1 °C) and in a humid environment of (50 ± 10%) during the experiment. The artificial day/night environment was provided with a lighting regime of 12-hour cycles.
Seven of the rats were placed in the control groups and 30 in the three experimental groups (G1, G2 and G3). A single dose of 15 Gy RT was applied to the head and neck area of the 10 rats in G1 group. A single dose of 15 Gy RT was applied to the head and neck area of the G2 group, but this group received a daily dose of 100 mg/kg of water-soluble propolis intraperitoneally for two weeks in the period following RT. G3 group was also given a single dose of 15 Gy RT in the head and neck area and received a daily dose of 200 mg/kg of water-soluble propolis intraperitoneally for two weeks in the period following RT.
Irradiation of Animals
RT implemented as part of the study was applied using Varian Clinac DHX 5776 linear accelerator 2014 (Varinak Medical Systems, Palo Alto, CA, USA). The application was carried out under the supervision of Radiation Oncology Department of Tokat Gaziosmanpasa University. After stabilizing the head and neck of the rats, computed tomography images were taken under anesthesia. The brain and eye parts of the rats were preserved and the targeted area was determined by 3D-conformal RT technique using 6 MV photon energy in two areas. The 30 rats in the experimental group were given a single fraction of 15 Gy RT in a single plan and in the same position.
Administration of Propolis
In our study, liquid propolis extracts obtained from SBS Scientific Bio Solutions Co. (Istanbul Technical University, 34469 Istanbul, Turkey) was used. G1 group had only RT while G2 and G3 groups were administered daily 100 and 200 mg/kg, respectively, liquid propolis extract intraperitoneally for two weeks. At the end of the study, there were seven rats in the control group, while one of the rats in Groups G1, G2 and G3 died, and data was obtained from nine rats in each of these groups.
At the end of the second week following RT, rats in experimental and control groups were sacrificed by cervical dislocation following high dose anesthetic injection. Then, the nasal regions of rats in all groups were excised and subjected to histological examination and analysis procedures.
Histological Procedures
Obtaining tissues and tissue tracking
The upper jaws of the rats were excised along with the nasal region and directly subjected to fixation in 4% buffered neutral formalin solution for 48 hours. Then, they were subjected to post-fixation in a decalcification solution with EDTA for up to four weeks. These tissues underwent routine histological tissue follow-up and embedded in paraffin blocks. From the samples in the paraffin blocks, 5-?m sections were taken using microtome and placed in microscope slides.
Hematoxylin-eosin staining
The 5-?m tissue sections taken from nasal region tissues, fixed with neutral formalin and embedded in paraffin blocks were incubated in an oven at 60 °C. They were deparaffinized via passing through xylene (3 x 5 min) and rehydrated by placing in each of the 100%, 96%, 90%, 80%, 70% alcohol series consecutively for five minutes each. The sections were incubated in hematoxylin for 10 minutes, then washed in running water for five minutes, immersed in acid alcohol and washed again in running tap water. Afterwards, they were stained in eosin dye solution for three min and placed in distilled water. In order to remove the excess dye, distilled water was replaced a few times. Then the sections were immersed in 80 and 90% alcohol solutions, and incubated in 96% alcohol for one minute and in absolute alcohol for two minutes in a row. After three separate xylene series, tissue sections were placed on a microscope slide, entellan was dropped onto them, and a cover slip was closed on the slides. Thus, hematoxylin-eosin-stained nasal tissue preparations were ready for microscopic analyses.
Histopathological analyses
Preparations were examined using a research light microscope and based on the criteria for inflammation score in Table 1. Inflammation scoring was performed on the mucosal parts of the vomeronasal organ, nasal septum, olfactory field and conchae in the nasal region for each rat. The analyses were carried out on an average of 5-6 consecutive sections for each rat. These preparations were examined histologically with a light microscope (Nikon Eclipse, Japan) in the Research Laboratory of Histology Embryology department, and inflammation scoring was obtained in five randomly selected areas (400 x magnification). The analyses were carried out blindly by a histologist who was unaware of the study groups. The average values of the results were calculated and statistical comparisons were made among the groups.
Table 1: Inflammation grading scale
Histomorphometric analyses
Histomorphometric analyses were performed on nasal tissue preparations using a NIS-Element software (Hasp ID: 6648AA61; Nikon) integrated to the light microscope (Nikon Eclipse, Japan). For this purpose, the epithelial tissue thickness of the nasal septum respiratory mucosa area, the olfactory mucosa area and the concha mucosa of the nasal mucosa, submucosa (lamina propria) thickness and total mucous thickness were measured at micrometric level under 40X magnification (Figure 1). The measurements were made on the same parts of the involved nasal mucosa in each rat. The average values of the measurements of the rats in each group were calculated for each parameter, and statistical comparisons were made among the groups.
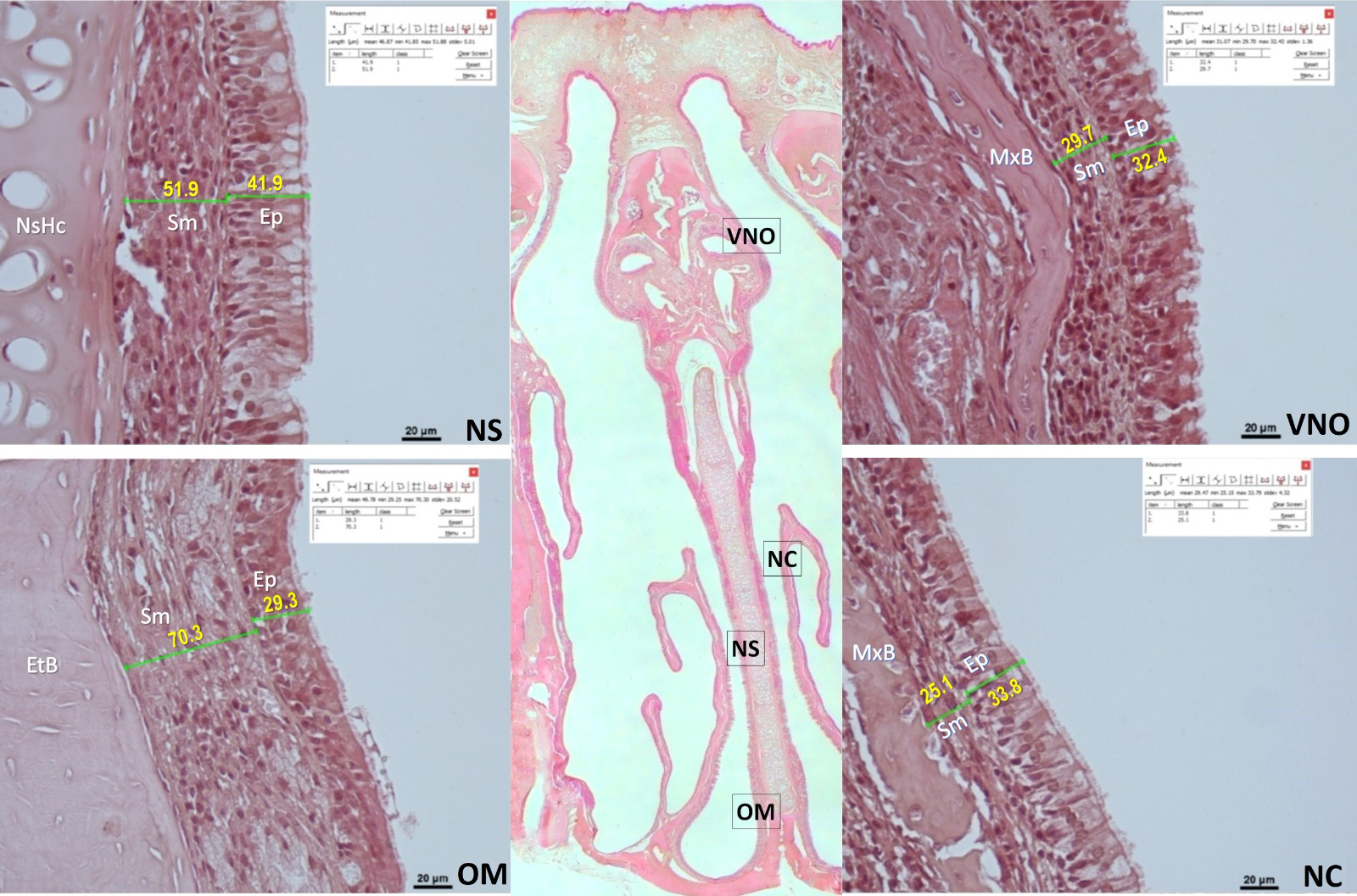 Büyütmek İçin Tıklayın |
Figure 1: Representative histological images of histomorphometric measurements in the control group. The big picture at the center is the microscopic image (HE, 4X) showing all nasal mucous sections together in the coronal plane. The images on the next are magnified images of the relevant parts of the nasal mucosa that were analyzed. These four lateral images are vomeronasal organ (VNO), nasal concha (NC), nasal septum (NS), and olfactory mucosal region (OM). The unit of length measurement is µ ,m. Ep: epithelium, Sm: submucosa, NsHc: nasal septum hyaline cartilage, MxB: maxilla bone, EtB: ethmoidal bone (HE, scale bar: 20 µm). |
Statistical Analyses
Statistical analyses of One-Way ANOVA and Post Hoc were performed using IBM SPSS-26 Windows statistical software. After the homogeneity test of the variances, the comparison of the inflammation scores were made with Tamhane test and the comparison of each nasal mucous parameter measurement results was made with Tukey HSD tests. The average values of the groups were expressed as mean ± standard error of the mean (SEM). P < 0.05 was considered statistically significant.
Results
Inflammation findingsThe analyses of hematoxylin-eosin stained preparations based on the criteria of the inflammation scale showed that the inflammation score was lowest in the control group and highest in G1 group which was given radiation only. In the G3 group, the inflammation score decreased to a level comparable to that of the control group, and was significantly different from those of G1 and G2 groups (p < 0.05). Inflammation in the G2 group was similar to G1 group (p > 0.05) (Figure 2).
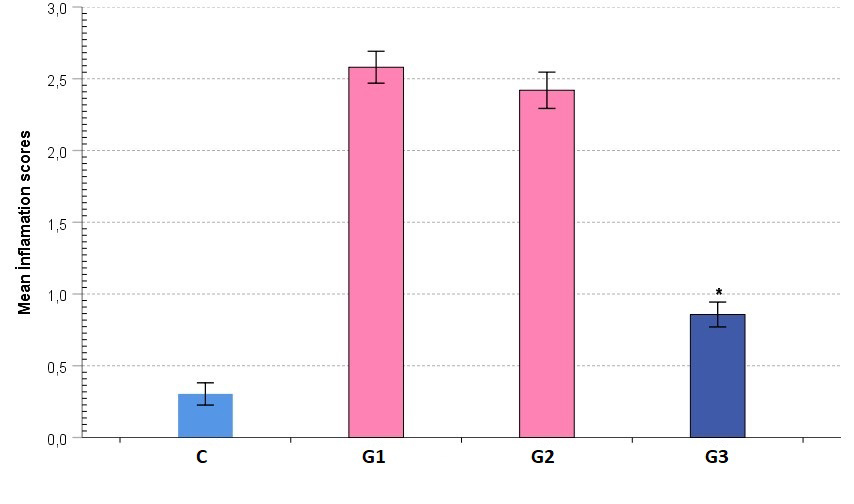 Büyütmek İçin Tıklayın |
Figure 2: Comparative graphical image of average group inflammatory scores. The colors of the bars show statistical differences. |
Histomorphometric findings
The general microscopic appearance of the nasal mucosa in the control group had normal tissue characteristics while in the G1 group, cellular damages in the epithelial layer, epithelial deformities and irregularities, edema areas in lamina propria, vascular dilatations, congestion and intense inflammatory cell infiltrations were notable. In the treatment groups, it was observed that the epithelium was reorganized and the histopathological damages were somewhat alleviated (Figure 3).
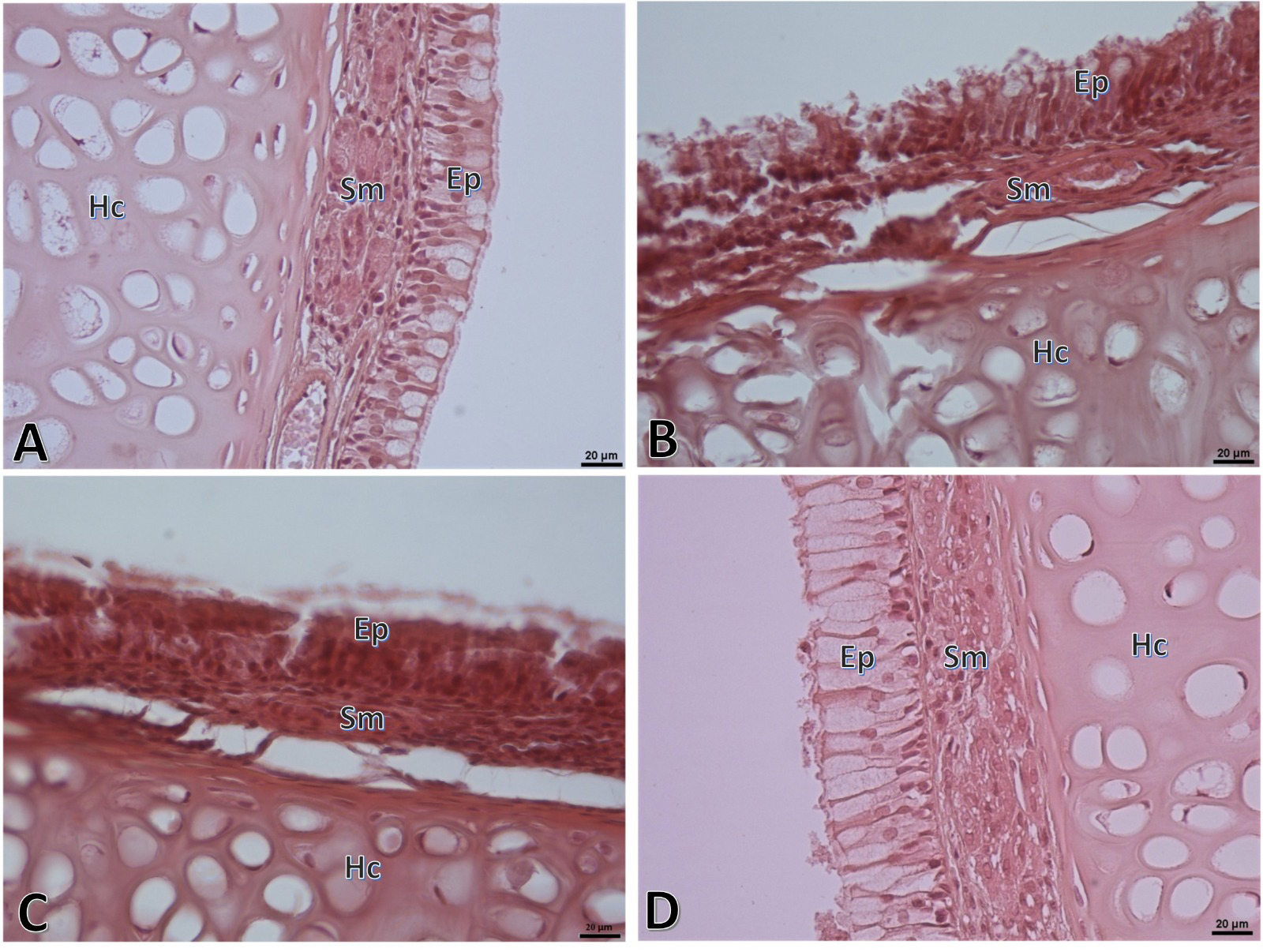 Büyütmek İçin Tıklayın |
Figure 3: Representative histopathologic microscopic images of respiratory mucous part of the nasal septum region of the nasal mucosa. Normal appearance is observed in the control group (A) while considerable tissue damage and inflammation are observed in the G1 group (B). Tissue damage and inflammation, though slightly alleviated, are observed in the G2 group (C). In the G3 group (D), considerably reduced tissue damage and inflammation are noted. Ep: Epithelial, Sm: Submucosa, Hc: Hyaline cartilage. (HE, Scale bar: 20µ). |
Epithelial thickness in the nasal septum respiratory area of the nasal mucosa was highest in the control group followed by the G3, G2 and G1 groups in decreasing order. Submucosa and total mucous thicknesses were highest in the G1 group. However, the differences among the groups for these measurements were not significant (p > 0.05, Figure 4).
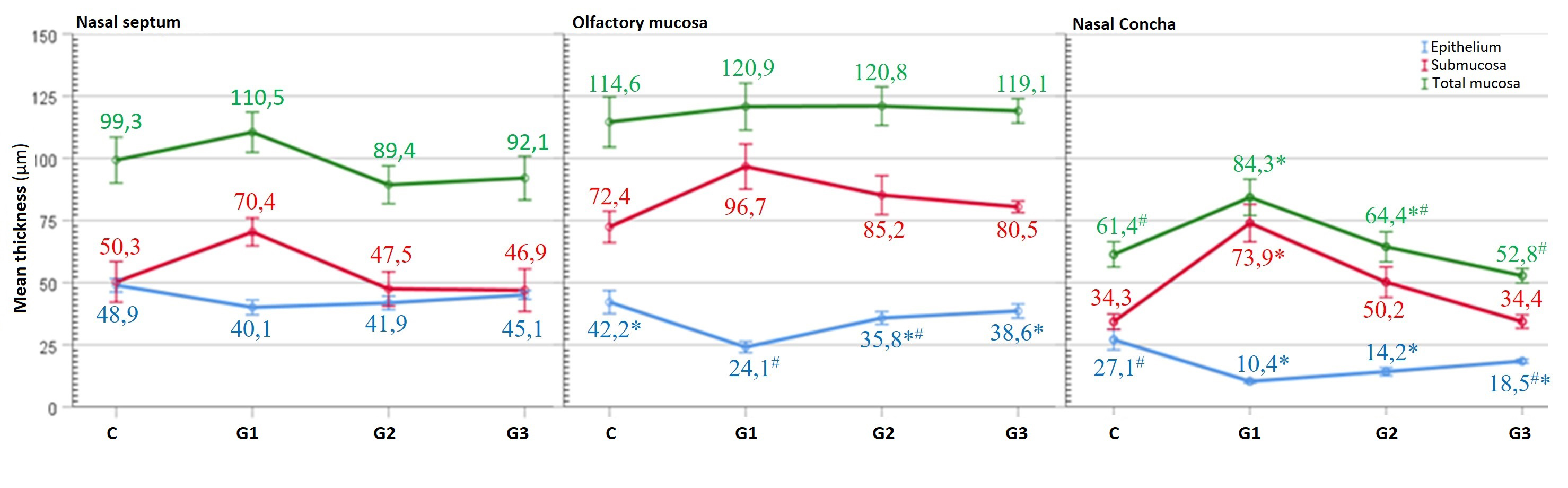 Büyütmek İçin Tıklayın |
Figure 4: Graphical comparative image of the group average values of the histomorphometric measurements of the nasal septum, olfactory region and concha parts respectively. The same symbols on the data of parameters indicate that the differences are not significant (p > 0.05). |
The thicknesses of the submucosa and total mucous in the nasal mucous olfactory region were similar (p > 0.05). However, the olfactory epithelial thickness was highest in the control group followed by the G3, G2 and G1 groups. The control, G3 and G2 groups were similar (p > 0.05). G1 was similar to G2 (p > 0.05) and these two groups were significantly different from others (p < 0.05, Figure 4).
The epithelial thickness of the concha region was highest in the control group which was followed by G3, G2 and G1 groups in decreasing order. Statistically, G1, G2, and G3 were similar to each other while the control group was similar to G3 (p > 0.05) and different from others (p < 0.05). The submucosa thickness in the concha area was highest in the G1 group and this difference was significant (p < 0.05). G1 group was followed by G2, G3 and control groups in decreasing order, but these differences were not significant (p > 0.05). The G1 group had the highest total mucous thickness in the concha region, which was statistically similar to G2 (p > 0.05) but significantly different from other groups (p < 0.05, Figure 4).
Discussion
Primary or adjuvant RT is an important treatment method that can be used in local control of head and neck carcinomas. RT is given to the targeted area but cannot distinguish between normal and diseased tissue, so it can damage healthy tissue. Under normal conditions, there is a balance between prooxidant and antioxidant systems in the organism. However, due to ionized radiation, free radicals such as superoxide radicals, hydrogen peroxide and hydroxyl radicals appear, and therefore, this balance is disturbed and oxidative stress is triggered. Free oxygen radicals negatively affect membrane lipids, proteins, nucleic acids and DNA structure, and cellular degradation begins as a result.[14,15] Inflammation is also triggered due to IL-1, IL-6 and TNF-?, proinflammatory cytokines released from cells after oxidative stress elicited by radiation damage.[16] Due to oxidative stress and triggered inflammation as a result of RT applied to the nasal, paranasal and nasopharyngeal region, patients may have complications such as mucositis, rhinorrhea, adhesion, edema and rhinosinusitis, and patient's quality of life decreases considerably.[17]Kamel et al.[18] examined 32 patients who received RT due to nasopharynx carcinoma which did not relapse. They evaluated 23 of these patients before and after RT. Nine patients were evaluated after RT. They showed that mucociliary clearance (MCC) testing in patients continued to deteriorate continuously up to the sixth month after RT, and then became stable. In the early post-RT period (two-six weeks), hyperemia, edema, macerated mucosa and discharge were observed in nasal endoscopy while crusting, scar formation, adhesions, atrophic conchae, ostium expansion (especially in the maxillary and sphenoid sinus) and choanal stenosis/atresia were observed in late period. Likewise, post-RT tomographic examinations showed an increase in sinusitis scores. MCC testing is important in that it gives information regarding the function of the nasal epithelium. Epithelial damage and ongoing sinusitis are the main factors responsible for prolonged MCC.[18]
A survey of literature yielded no studies except one[17] examining black cumin oil (Nigella sativa [NS]) to prevent the side effects of RT on the nasal and paranasal sinus mucosa. In the study,[17] a total of 18 rats divided into three groups of six were examined, and one drop of saline (0.05 mL) was dropped into each nostril of rats in Group 1 on the first, second and third days. A single dose of 40 Gy RT was given to the nasal and paranasal regions of rats in Group 2 and Group 3. A drop of saline was also dropped into each nostril of the rats in the Group 2 on the first, second and third days. Cold pressed NS (0.05 mL) was applied to each nostril of the rats in Group 3 on the first, second and third days. Fourteen days after radiation application, the nasal mucosal tissues were excised for histopathological examination. Nasal mucosa damage was classified as vascular dilatation, inflammatory cell infiltration, superficial erosion and formation of exudates according to the severity of the damage. No mucositis was observed in Group 1. Of all parameters studied, only the "superficial erosion" was significantly different between Group 2 and Group 3 (p < 0.05). In other microscopic examinations, data of the group treated with NS were better than Group 2, but the difference was not significant (p > 0.05). According to the data of the study, local application of NS was found to be effective in the treatment of acute nasal mucositis due to RT.[17] Because of its antioxidant, cytoprotective, anti-inflammatory and antineoplastic effects, NS was shown to be effective on damage to the nasal and paranasal mucosa.[17]
Although studies were carried out on the usefulness of propolis in preventing and healing the harmful effects of radiation in different tissues,[16,19,20] no studies have been found in the literature dealing with its effect on the nasal region. Benderli et al.[19] applied propolis at 50 mg/kg and 100 mg/kg doses to rats exposed to 18 Gy radiation. In parallel with the increased dose, healing effect of propolis application was shown on both the overall body weight of the rat and the severity of oral mucositis.[19] Guler et al.[16] applied 100 mg/kg and 200 mg/kg propolis to rats exposed to 15 Gy radiation and observed a dose-dependent beneficial effect of propolis on tongue and overall body weight.[16] Khayyal et al.,[20] on the other hand, used five groups of rats, one control and four treatment groups which received 8 Gy RT, and examined the intestinal mucosae of rats exposed to radiation. Three of the groups receiving radiation were given 450, 650 and 850 mg/kg doses of oral propolis. Seventy-two hours after RT, the rats were sacrificed, and small intestines were excised and examined. Serum MPO activity and TNF-? levels of the group receiving 850 mg/kg of oral propolis were significantly lower compared to other radiation exposed groups.[20] It was emphasized that the application of high dose propolis is important in protecting the small intestine from radiation damage.[20] El-Anwar et al.[21] examined the effect of propolis on the mechanically traumatized nasal region in a total of 18 rats divided into three groups. The trauma was created in the right-side nasal mucosae of all rats with the help of a brush. No treatment was administered to the first group. Gum tragacanth (suspension agent for propolis) was applied to the second group while the third group received propolis. The first group received no treatment except for routine care for 15 days. The second group was given a single dose of gum tragacanth per day orally at 5 mg/kg rate for 15 days. The third group received a single dose of propolis suspension per day orally at 100 mg/kg rate for 15 days. Tissue samples obtained at the end of the study were examined under the light microscope after staining with hematoxylin-eosin. Ulceration was not observed in the group treated with propolis and the intensity of the inflammation was lower compared to the control and gum tragacanth groups. Loss of goblet and ciliary cells was also lower in the group treated with propolis than in the other two groups. Propolis was shown to reduce inflammation and improve healing in rat nasal mucous damage.[21]
In the present study, RT was shown to increase inflammation in the nasal mucosa, cause epithelial damage, and lead to an increase in the thickness of the submucosal region and total mucous thickness. It was observed that propolis played a healing role for all these side effects in a dose dependent manner.
Our study is the first to examine the effect of propolis on the nasal mucosa of radiation-exposed rats. It was shown histopathologically and histomorphometrically that the application of systemic propolis reduces inflammation and increases healing in the radiation-exposed rat nasal mucosa in a dose dependent manner. However, it could be useful to support this data with future experimental and clinical trials, including more detailed and comprehensive studies.
Funding: There is no funding for this study.
Conflict of Interest: The authors declare that they have no conflict of interest.
Reference
1) Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55: 74. [ Özet ]
2) Gregoire V, Lefebvre J, Licitra L, Felip E, EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21 (Suppl. 5): v184-v186. [ Özet ]
3) Germano F, Melone P, Testi D, Arcuri L, Marmiroli L, Petrone A, Arcuri C. Oral complications of head and neck radiotherapy: prevalence and management. Minerva Stomatol. 2015; 64: 189-202. [ Özet ]
4) Riva G, Boita M, Ravera M, Moretto F, Badellino S, Rampino M, Ricardi U, Pecorari G, Garzaro M. Nasal cytological changes as late effects of radiotherapy for nasopharyngeal cancer. Am J Rhinol Allergy. 2015; 29:e41-e45. [ Özet ]
5) Praetorius NP, Mandal TK. Alternate delivery route for amifostine as a radio-/chemo-protecting agent. J Pharm Pharmacol. 2008; 60: 809-815. [ Özet ]
6) Al-Ansari S, Zecha JA, Barasch A, de Lange J, Rozema FR, Raber-Durlacher JE. Oral mucositis induced by anticancer therapies. Curr Oral Health Rep. 2015; 2: 202-211. [ Özet ]
7) Cuba LF, Salum FG, Cherubini K, de Figueiredo MAZ. Antioxidant agents: a future alternative approach in the prevention and treatment of radiation induced oral mucositis? Altern Ther Health Med. 2015; 21: 36-41. [ Özet ]
8) Talas ZS, Ozdemir I, Ciftci O, Cakir O, Gulhan MF, Pasaoglu OM. Role of propolis on biochemical parameters in kidney and heart tissues against L-NAME induced oxidative injury in rats. Clin Exp Hypertens. 2014; 36(7):492-496. [ Özet ]
9) Orsolic N, Sver L, Terzic S, Basic I. Peroral application of water-soluble derivative of propolis (WSDP) and its related polyphenolic compounds and their influence on immu-nological and antitumour activity. Vet Res Commun. 2005; 29:575-93. [ Özet ]
10) Grunberger D, Banerjee R, Eisinger K, Oltz EM, Efros L, Caldwell M, Estevez V, Nakanishi K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia. 1988; 44(3):230-232. [ Özet ]
11) Astani A, Zimmermann S, Hassan E, Reichling J, Sensch KH, Schnitzler P. Antimicrobial activity of propolis special extract GH 2002 against multidrug-resistant clinical isolates. Pharmazie. 2013; 68(8):695-701. [ Özet ]
12) Sforcin JM. Biological properties and therapeutic applications of Propolis. Phytother Res. 2016; 30: 894-905. [ Özet ]
13) Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011; 133: 253-260. [ Özet ]
14) Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS. 2007; 115:81-103. [ Özet ]
15) Cooke MS, Olinski R, Evans MD. Does measurement of oxidative damage to DNA have clinical significance? Clin Chim Acta. 2006; 365:30-49. [ Özet ]
16) Avci GG, Erdim I, Ozmen ZC, Gevrek F, Colak S, Demirsoy MS, Bozkurt H. The effect of systemic application of propolis on tongue damage and oral mucositis in rats exposed to radiation. Eur Arch Otorhinolaryngol. 2022; 279(2):1043-1052. [ Özet ]
17) Canakci H, Yilmaz AAS, Canpolat MS, Seneldir H, Kir G, Eris AH, Mayadagli A, Oysu C. Evaluation of the Effect of Topical Application of Nigella sativa on Acute Radiation-Induced Nasal Mucositis. J Craniofac Surg. 2018; 29(3):e279-e282. [ Özet ]
18) Kamel R, Al-Badawy S, Khairy A, Kandil T, Sabry A. Nasal and paranasal sinus changes after radiotherapy for nasopharyngeal carcinoma. Acta Otolaryngol. 2004; 124(4):532-5. [ Özet ]
19) Cihan BY, Deniz K. Efect of propolis against radiation-induced oral mucositis in rats. J Ear Nose Throat. 201; 21:32-41. [ Özet ]
20) Khayyal MT, Abdel-Naby DH, El-Ghazaly MA. Propolis extract protects against radiation-induced intestinal mucositis through anti-apoptotic mechanisms. Environ Sci Pollut Res Int 2019; 26:24672-24682. [ Özet ]
21) El-Anwar MW, Said Abdelmonem S, Abdelsameea AA, AlShawadfy M, El-Kashishy K. The Effect of Propolis in Healing Injured Nasal Mucosa: An Experimental Study. Int Arch Otorhinolaryngol. 2016; 20(3):222-5. [ Özet ]




