PROTECTION AGAINST AMINOGYCOSIDE OTIC DROP-INDUCED OTOTOXICITY BY A SPIN TRAP(PBN): CHRONIC EFFECTS
2The University of Kentucky College of Medicine, Otolaryngology Head and Neck Surgery, Kentucky, ABD
Summary
Objectives: To investigated the long-term spin trap protection against aminoglycoside otic drop-induced ototoxicity.Material and Methods: We evaluated the ability of intraperitoneal α-phenyl-tert-butyl-nitrone (PBN) or 0.9% saline applications to reduce the chronic effects of topical aminoglycoside otic drops (AD) or artificial perilymph (PER) treatment on cochlear microphonic (CM), compound action potential (CAP) and 2F1-F2 distortion product otoacoustic emission (DPOAEs) sensitivities in three groups of guinea pigs. (n=5/group): a) saline+PER (controls), b) saline+AD and c) PBN+AD.
Results: Relative to control values, saline+AD group mean CM and CAP values were severely degraded at all frequencies (p<.05). PBN+AD group mean losses were significantly (p<.05) smaller than those of the saline+AD group for CAP and CM at 8, 12, 16 and 24 kHz, indicating that PBN provided protection against topical AD induced damage. Mean 2F1-F2 DPOAE values decreased to below background levels at all frequencies in groups receiving ADs, irrespective of PBN treatment.
Conclusion: These findings suggest that free radical mediated events are partially responsible for, and that DPOAEs are very sensitive to, AD induced chronic ototoxicity in guinea pigs.
Introduction
Otic drops containing neomycin, polymixin B and Hydrocortixone are commercial preparations widely prescribed for the treatment of middle ear infections. Topical application of these aminoglycoside based drops (ADs) has not been shown to induce a high rate of human auditory dysfunction (1,2,3), However, the use of them engenders concern (4) because systemic aminoglycoside administrations result in clinically observable hearing losses, and because topical application of ADs, or of neomycin or polymixin B individualy, results in permanent cochlear disruption in rodents (1,5).A major advance in the treatment of experimentally-induced ototoxicities is the use of free radical scavengers (4-10). Free radicals have been directly shown to disrupt cochlear structure (11) and function (12), and free radical scavengers have been demonstrated to both reduce cochlear free radical generation (9,13) and to protect the cochlea from the damage which follows various ototoxicant exposures (4-10). Specific to AD induced ototoxicity, our laboratory has demonstrated that combined topical and systemic pre-administration of α-phenyl-tert-butyl-nitrone (PBN), a spin trap for organic and oxygen-derived radical species (10,14), provides near total protection against the acute disruption of cochlear compound action potential (CAP) and cochlear microphonic (CM) sensitivities that follows topical AD administration in the guinea pig (15). That study, however, left unresolved the issue of whether PBN actually prevented, or simply delayed, the ototoxic effects of ADs.
The present investigation examined the ability of intraperitoneal (i.p) application of PBN to prevent the chronic cochlear dysfunction that results from single dose, transtympanic AD administration in the guinea pig. It was postulated that if chronic AD-induced ototoxicity is mediated by free radical generation, then PBN would reduce the permanent effects of AD administration on CM, CAP and 2F1-F2 distortion product otoacoustic emission (DPOAEs) sensitivities.
Methods
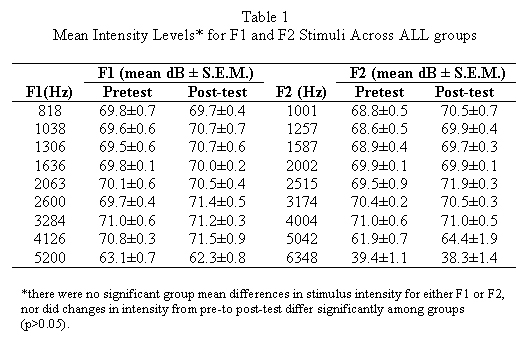
Subjects were 15 pigmented guinea pigs (2/NCR, National Cancer Institute; 250-350 gm) housed in an AAALAC (American Association for Accreditation of Laboratory Animal Care) accredited vivarium. Each animal was individually brought into the laboratory, anesthetized (ketamine, 35 mg/ kg; xylazine, 3 mg/ kg; i.m.) and placed on a warming table in a double-walled sound attenuated booth (I.A.C. industrial). The animals head was rigidly stabilized, and its right external ear was otoscopically examined. A customized plastic speculum mounted on a manipulator was inserted into the external auditory meatus, and a pediatric probe fitted with a Styrofoam collar was sealed into the speculum. 2F1-F2 DPOAE measurements were then recorded using a standart OTOdynamics model ILO92 (USA) device, which presented nine F1-F2 acoustic stimulus combinations: 818-1001, 1038-1257, 1306-1587,1636-2002, 2063-2515, 2600-3174, 3284-4004, 4126-5042, and 5200-6348 Hz. Mean F1 and F2 intensities are given in Table 1. Standart Distortion Product-grams were computer generated and stored for analysis.A given animal was then administered one of three drug combinations in which PBN (60mg/ kg) or an equal volume of 0.9 % saline (1.0ml), was administered i.p., followed in 10 minutes by transtympanic application of artificial perilymph (PER) or AD (50µ ,l). PER was prepared as previously described (9,11,12,13). AD was a commercially available solution (Schein Pharmaceuticals, Florham Park, NJ) which contained neomycin sulfate (3.5 mg base/ ml), polymyxin B sulfate (10.000 U/ml), and hydrocortisone (10 mg/ml). The three exposure groups (N=5/ group) defined by drug treatment conditions were: a) saline+PER (controls), b) saline + AD and c) PBN+ AD. Following exposure, the animal was kept warm and prone in a holding cage until awake, and then returned to the vivarium. Post-testing of auditory function occurred two weeks later.
Recording of CM and CAP Responses
At the end of two weeks, the quinea pigs were returned to the laboratory, anesthetized (urethane,1.5 g/kg, i.p.), placed upon the warming platform, and 2F1-F2 DPOAE values were again recorded. Standart ventral approach surgery was performed, as previously described (12,15), and the middle ear was inspected for signs of effusion, inflammation and any other signs of abnormality. Only one animal (in the PBN+AD group) showed scant signs of middle ear effusion, but no grossly abnormal middle ear mucosa was noted. CAP thresholds and 1.0µV RMS (root mean square) CM isopotential curve values were recorded by a second experimenter, blinded to the animals group assignment(Interstellar,Ann Arbor,MI,USA). Stimuli were 2, 4, 6, 8, 12, 16, 20, 24, 30, 35 and 40 kHz pure tone frequencies, presented closed-field to the external auditory meatus by a ½ inch pressure microphone (ACO Pacific model 7013,USA) housed in a plexiglass speculum. CM and CAP responses were recorded from a Teflon insulated silver ball electrode (0.11 mm wire, A-M Systems, Inc.) placed on the the round window membrane, referenced to a Ag/AgCl electrode grounded in neck muscle. Rectal temperature was maintained at 38˚ C (± 1°), and a d.c lamp prevented the cooling of the bulla. This protocol adheres to policies advocated by the National Research Council Gide for the Care and Use of Laboratory Animals, and was approved by the University of Kentucky Institutional Animal Care and Use Committee.
CM and CAP Stimulus and Recording Parameters
Acoustic stimuli for determining CAP thresholds and for generating 1.0µV RMS CM isopotential curves were pure tone bursts (10 msec duration, 1.0 msec rise/fall sidebands;9/sec).and continuous pure tone, respectively. Signals were generated by an oscillator, amplified, and then shaped and attenuated in single dB steps under computer control (Interstellar, Ann Arbor, MI,USA) by Wilsonics PATT and BSIT devices. Stimulus intensities at the tympanic membrane were calibrated using a 12 inch microphone with a 1.0 mm (O.D.) probe attachment, which was guided through a channel in the speculum to within 1.0 mm of the tympanic membrane. Electrophysiological responses were amplified 1000 x by a Grass model P-15 differential amplifier, band-pass filtered at .3 and 50 kHz for CM and at .3 and 3 kHz for CAP, and routed to a lock-in amplifier (Stanford Instruments )and a triggereble oscilloscope. CAP threshold for a given frequency was defined as the stimulus intensity which produced the minimally detectable N1 response upon visual inspection. Objective CM isopotential curves were computer generated from the stimulus intensities which elicited a 1.0µV RMS sinusoidal response for each frequency.
Statistical Analyses
2F1-F2 DPOAE values were subjected to a three-way analysis of variance, using F1-F2 combination (i.e., 818-1001, 1039-1257, etc) and time (pre- vs. post-exposure) as within subject factors, and drug combination as the between group variable. A four way repeated measures analysis of variance of DPOAE stimulus intensities, using drug combination as the between group factor and time, F1-F2 combination and F category (F1 vs.F2) as within group factors, was utilized to ensure that response changes were not due to differences in stimulus intensities between groups, or from pre-to post-testing. In 2F1-F2 DPOAE Illustrations, Noise (2sd), the standart noise floor averaged across all recordings, is represented because it was very stable (S.E.M values ranging only between .22 and .35 dB) and did not differ significantly among groups (F(2,23)=2.97, P>.05). CM isopotential and CAP threshold values were analyzed separately by two-way analyses of variance utilizing frequency as a repeated measure and drug treatment as the grouping variable. In all cases, the Tukeys honest significant differences test provided post hoc analyses of specific pair-wise group mean differences; the criterion level for statistical significance was p<0.05. Probability values reported for a range of frequencies refer to the largest individual p-value obtained.
Results
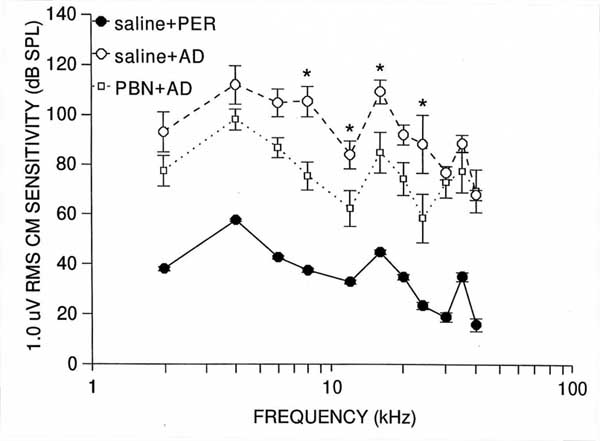
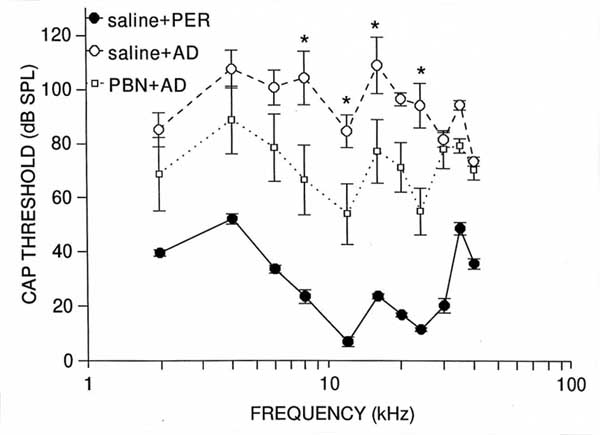
Compound Action PotentialThe analysis of CAP threshold data revealed that the main effects of group (F(2,12)=43.01, p<.0001), and frequency (F(10,120)=10.26, p<.0001), and the group x frequency interaction (F(20,120)=3.32, p<.0001) were significant. Saline + AD group mean values differed significantly from those of controls at every frequency (p<0.001), with losses averaging 82 dB between 8 and 24 kHz (see Figure 1). These results confirm that AD administration disrupted CAP function. PBN+ AD group mean values also differed significantly ( p<.05) from those of controls at every frequency expect 30 and 40 kHz. However, mean CAP threshold decrements in the PBN+ AD group were not as great as those of the saline +AD group at any frequency, and were significantly less at 8 (p<.001), 12 (p<.05), 16 (p<.05) and 24 (p<.001) kHz. These groups mean differences (i.e protection) averaged 33 dB in the 8-24 kHz range (Figure 1). Thus, i.p. PBN provided partial protection against chronic AD-induced CAP threshold decrements.
Cochlear Microphonic
Results of the analysis of CM data generally paralleled those of CAP, yielding significant main effects of group (F(2,12)=54.9, p<.0001), and frequency (F(10,120)=26.96, p<.0001), and a significant group x frequency interaction (F(20,120)=2.08, p<.01). Saline +AD group means differed significantly (p<.001) from those of controls at every frequency, and averaged 96 dB in the 8-24 kHz range, indicating that AD application also disrupted CM function (Figure 2). PBN + AD group mean values also differed significantly (p<.001) from those of controls at every frequency, averaging 71 dB between 8 and 24 kHz. However, relative to the saline+AD group, the PBN+AD group means showed less CM disruption at evey frequency, and significantly so at 8 (p<.001), 12 (p<.05), 16 (p<.01) and 24 (p<.001)kHz. Between 8 and 24 kHz, the average amount of protection (i.e., the difference between PBN +AD and saline +AD group values ) was 25 dB. These data indicate that i.p. administered PBN provided partial protection against AD-induced CM disruption, again, centered in the mid-to-high portion of the quinea pigs auditory range.
2F1-F2 Distortion Product Otoacoustic Emissions
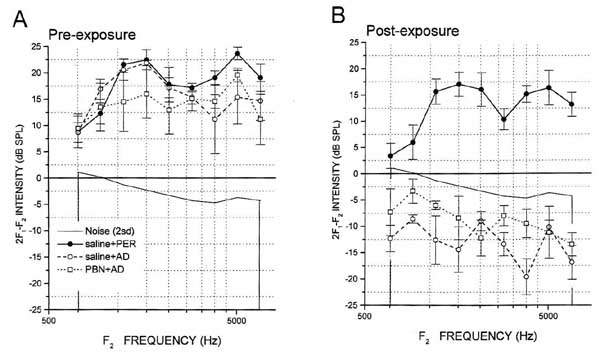
F1 and F2 stimulus intensity levels did not differ significantly (p> 0.05) among groups as a main effect (F(2,10)=0.18, p=0.83; see Table 1) or as a function of time (pre- vs. post-test; F(2,10)= 0.54,p=0.60 ). 2F1-F2 DPOAEs were clearly recordable in all groups at baseline (Figure 3). The analysis of variance yielded significant main effects of group (F(2,10)=25.06,p<.001), time (F(1,10)=178.78, p<.0001), and frequency (F(8,80)=5.72, p<.0001), as well as significant group x time, (F(2,10)=24.43, p<.001) and group x frequency (F(16,80))=3.13, p<.001) interactions. Post hoc analysis revealed that there were no significant group mean differences in baseline 2F1-F2 DPOAE values, at any frequency (p>.05). At post-exposure, saline+PER control group mean post-exposure values remained robust and did not differ significantly (p>.05) from their pre-test values at any frequency (see Figure 3). However, in all animals of the other groups, mean 2F1-F2 DPOAE values for each frequency fell to below background. Statistically, the saline+AD groups mean post-exposure scores differed significantly from their pre-exposure counterparts at each F1-F2 combination (p<.001), and from saline +PER group post-exposure scores at every frequency combination expect 1038-1257 Hz (p<.05). The PBN+ AD groups mean post-exposure scores differed from their pre-exposure values at every frequency combination expect 1038-1257 Hz (p<.001), and from saline +PER group post-exposure values at every frequency combination from 1306-1587 to 5200-6348 Hz (p<.01). Therefore, PBN did not prevent the AD induced decrement of DPOAE responses. Figure 3 illustrates group mean pre- and post-exposure 2F1-F2 DPOAE values.
Discussion
The present results support previous findings that topically applied aminoglycoside-based otic drops, as utilized routinely in clinical practic, induce a chronic loss of CM and CAP function in rodents (1,2,5,16,17). These results extend the literature, by showing that 2F1-F2 DPOAEs are very sensitive, even more so than CM and CAP, to AD ototoxicity. More importantly, this is the first report to demonstrate that free radical scavengers, and in particular, a single i.p. PBN pre-treatment, can significantly attenuate the loss of mid-to-high frequency CM and CAP sensitivities withronically topical AD administration. Because CM and CAP responses could not be evoked in some animals by even the highest intensity stimuli produced by our equipment, these findings conservatively estimate the amounts of both AD-induced hearing loss and PBN-related protection. These data suggest that AD induced ototoxicity is partially mediated by free radical generation, and free radical scavenging may aid in preventing such ototoxicity.The ability of PBN to attenuate AD induced ototoxicity is consistent with the growing literature that implicates free radical generation in the ototoxicities of various aminoglycosides. Polymyxin B is ototoxic (17) and almost certainly contributed to the CM, CAP and 2F1-F2 DPOAE losses seen in the AD exposed animals. However, there is no evidence that polymyxin B generates, or promotes the generation of, free radicals. Aminoglycoside antibiotics have been directly shown using electron paramagnetic resonance spectrometry to initiate or promote the metal catalyzed generation of oxygen-derived free radical species in cochlear tissues (9,13). This may partially occur through the conjugation of iron by aminoglycosides, as the use of iron chelators, which prevents hydroxyl radical production (9), also prevents aminoglycoside-induced damage (18). Aminoglycoside-induced free radical formation has been consistently demonstreated in other organ systems,(19-21) where mitochondrial iron release is associated with hydroxyl radical generation (20,21). Free radical scavengers reduce cochlear free radical generation (9,13), and prevent the toxic effects of aminoglycosides on structure and function in several organs, including the cochlea, both in vivo (7) and in vitro (8). Therefore, the improved outcomes of PBN exposed animals in the present study likely reflect the reduction of free radical generation associated with neomycin ( or neomycin potentiated by polymixin , rather than a decrement in polymyxin Bs separate toxic effects. It is not clear why the near total protection afforded by PBN acutely in the Hester et al. study (15) was not maintained at two weeks. One methodological difference that might account for this discrepancy is that, in the Hester et al. study, PBN was applied directly to the round window and administered i.p. Topical administration was not utilized in the present study because the PBN, or control solution (PER) could not be removed from the bulla, and so, would alter the middle ear AD concentration.
PBNs greater protective efficacy in the acute phase of ototoxicity suggests that free radical generation might play a larger role during the acute, post-AD exposure period, than it does later. However, because CM showed little disruption acutely, but later showed great disruption which was partially ameliorated by PBN, free radical generation and its consequences likely continue beyond the acute period. To the extent that the ototoxic effect of ADs is due to free radical mediated events, it is necessary for the scavenging agent to be available the entire time that free radicals are generated. Here, ADs remained in the middle ear. So, if they continued to produce free radical mediated damage after the spin trapping ability of PBN had been exhaused, then PBN would reduce the acute, but not the chronic, ototoxicity. A more frequent schedule of PBN administration might then increase the amount of protection. That the otic drops used here contain more than one ototoxic component further confounds the relationship between the acute and chronic toxicities. One component might induce toxicity via free radical generation, while another operates via a different mechanism. If these effects occur at differing rates, for example, if the effects of the component acting via non-radical mechanisms (e.g., polymyxin B) follow the generation of free radicals by the other component (e.g., neomycin), then free radical scavenging would be effective acutely, but not chronically. Further investigation is required to clarify all of these issues. However, it is clear that the auditory dysfunction seen at two weeks after AD administration is much more severe than that seen in the first hour. Even a single pre-administration of PBN near completely negates the acute ototoxicity (15), suggesting that, at this point in time, AD-induced effects are primarily mediated by free radical generation. Much less, although significant, protection remains at two weeks, when free radical generation may contribute less to the ototoxicity.
The results of the present study suggest that 2F1-F2 DPOAEs are exquisitely sensitive to the effects of ADs. 2F1-F2 DPOAE responses at two weeks could not be recorded in any animal that received topical AD application, irrespective of PBN administon. That 2F1-F2 DPOAE responses are totally lost when CM and CAP responses are still recordable suggests that DPOAEs may provide an excellent screening device for ototoxicity. Decrements in CM and 2F1-F2 DPOAE sensitivities indicate that outer hair cell dysfunction is among the chronic effects of topical AD treatment, and this is congruent with reports of outer hair cell loss days to weeks following exposure (1,5). Both CM and 2F1-F2 DPOAEs are believed to require outer hair cell functional integrity (22). While 2F1-F2 DPOAEs also rely upon descending innervation from the superior olivary complex, topically-applied ADs do not have access to the brainstem. The severe loss of CAP threshold sensitivity indicates that the synchronous afferent signals initiated by the inner hair cells and translated into action potentials of the type one spiral ganglion cells were also disrupted. However, the alterations indexed by reduced CM, CAP and 2F1-F2 DPOAE function could also be explained by stria vascularis dysfunction, with secondary disruption of inner and outer hair cell function. Further experimentation, including histological examination of the cochlea and the recording of endocochlear potentials, is required to determine which structures were primarily affected by ADs and protected by PBN.
In conclusion, topical AD administration induces a chronic cochlear dysfunction in the guinea pig that is reflected in altered CM, CAP and 2F1-F2 DPOAE sensitivities. Among these measures 2F1-F2 DPOAEs appear to be the most sensitive to AD ototoxicity. Some of these changes can be partially prevented by systemic administration of the spin trap, PBN, even though the near total of protection afforded acutely by PBN is not maintained two weeks after AD exposure. Advances in our understanding of the most efficacious scavenging agents and dosing schedules should increase the amount of protection that can be obtained by such means. Since topical application of ADs in rodents results in ototoxicity, it is the parent compounds in ADs , not metabolites produced in other organs, that are responsible for the cochlear dysfunction. Accordingly, it must be assumed that if ADs could access the cochlear fluids in humans, there would be the potential for damage to occur. Clinical reports of human AD ototoxicity are rare, and this may be due to differences between humans and rodents with respect to membrane permeability and round window niche position (1,2,3), however, AD administration might still pose a potential danger in patients with an abnormal cochlear anatomy or a perilymphatic fistula. Although the use of spin traps and other free radical scavengers to protect cochlear function and structure is a relatively recent area of study, this technology provides exciting potential for improving the outcomes of various ototoxicant exposures and for understanding the mechanisms underlying the effects of ototoxic agents in the auditory periphery.
Reference
1) Meyerhoff WL, Morzono T, Shaddock LC, Wright CG, Shea DA, Sikora MA. Tympanostomy tubes and otic drops. Laryngoscope 1983; 93:1022-27. [ Özet ]
2) Morzano T. Toxicity of ototopical drugs: animal modeling. Ann Otol Rhinol Laryngol 1990; 99: 42-45. [ Özet ]
3) Roland PS. Clinical ototoxicity of topical antibiotic drops. Otolaryngol Head Neck Surg 1994;110:598-602. [ Özet ]
4) Lundy LB, Graham MD. Ototoxicity and ototopical medications: a survery of otolaryngologists. Am J Otol 1993;14:141-146. [ Özet ]
5) Barlow DW, Duckert LG, Kreig CS, Gates GA. Ototoxicity of topical otomicrobial agents. Acta Otolaryngol ( Stockh) 1995;115:231-235. [ Özet ]
6) Ravi R, Somani SM, Rybak LP. Mechanism of cisplatin ototoxicity: Antioxidant system. Pharmacol Toxicol 1995;76:386-394. [ Özet ]
7) Garetz SL, Altschuler RA, Schacht J. Attenuation of gentamicin ototoxicity by glutathione in the guinea pig in vivo. Hear Res 1994;77:81-87. [ Özet ]
8) Garetz SL, Rhee DJ, Schacht J. Sulfhydryl compounds and antioxidants inhibit cytotoxicity to outer hair cells of a gentamicin metabolite in vitro. Hear Res 1994; 77:75-80. [ Özet ]
9) Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res 1996;98:116-124. [ Özet ]
10) Tuncel U, Clerici WJ, Jones RO. Differential ototoxicities induced by lead acetate and tetraethyl lead. Hear Res 2002;166:113-123. [ Özet ]
11) Clerici WJ, DiMartino DL, Prasad MR. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear Res 1995;84:30-40. [ Özet ]
12) Clerici WJ, Yang L. Direct effects of intraperilymphatic reactive oxygen generation on cochlear potentials. Hear Res 1996;101:14-22. [ Özet ]
13) Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct determination of reactive oxygen species generation by ototoxicants using electron paramagnetic resonance spectroscopy. Assoc Res Otolaryngol abstr. 1995;18: 80.
14) Carney JM, Floyd RA. Protection against oxidative damage to CNS by á- phenyl-tert-butyl-nitrone (PBN) and other spin-trapping agents: a novel series of nonlipid free radical scavengers. J Mol Neurosci 1991;3:47-57. [ Özet ]
15) Hester TO, Jones, RO, Clerici WJ. Protection against aminoglycoside otic drop-induced ototoxicity by a spin trap (PBN): I. Acute effects . Otolaryngol Head Neck Surg 1998; 119: 581-87. [ Özet ]
16) Ikeda K, Morizono T, Juhn SK. Cochleotoxcity of otic drops in the chinchilla: comparative study of Bestron and Cortisporin. Am J Otol 1991;12(6):429-34. [ Özet ]
17) Wright CG, Meyerhoff WL, Halama, AR. Ototoxicity of neomycin and polymyxin B following middle ear application in the chinchilla and baboon. Am J Otol 1987; 8(6): 495-99. [ Özet ]
18) Song B-B, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hear Res 1996; 94: 87-93. [ Özet ]
19) Nakajima T, Hishida A, Kato A. Mechanisms for protective effects of free radical scavengers on gentamicin-mediated nephropathy in rats. Am J Physiol 1994 ;266(3) :F425-31. [ Özet ]
20) Ueda N, Guidet B, Shah SV. Gentamicin-induced mobilization of iron from renal cortical mitochondria. Am J Physiol 1993; 265: F435-9. [ Özet ]
21) Walker PD, Shah SV. Evidence suggesting a role for hydroxyl radical in gentamicin- induced acute renal failure in rats. J Clin Invest 1988;81:334-41. [ Özet ]
22) Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear 1990;11(2):82-92. [ Özet ]