PERIPHERAL EOSINOPHILIA AND ITS RELATION WITH CT SCORES IN CHRONIC RHINOSINUSITIS
2Cumhuriyet Universitesi Tıp Fakültesi, Radyoloji ABD, Sivas, Türkiye
Summary
The purpose of this study was to quantify peripheral eosinophil count and percentage of eosinophils in white blood cell (WBC) count of the patients with chronic rhinosinusitis (CRS) and look for the correlation between the CT scores and peripheral eosinophilia.Peripheral eosinophil counts and the percentages of eosinophils in white blood cell count (WBC) were statistically compared with CT scores in chronic rhinosinusitis (CRS) patients. We also statistically analyzed the peripheral eosinophil counts and the percentages of eosinophils in WBC counts between CRS patients and healthy ones.
We found significant difference between CRS group and control group for peripheral eosinophil count, and percentage of eosinophils in WBC count (p=0.001 and p<0.001) respectively. We observed no correlation between CT scores and both peripheral eosinophil counts, or percentages of eosinophils in WBC counts (p=0.067and p=0.051) respectively.
Although eosinophil seems as the dominator cell of CRS patients in the periphery, peripheral eosinophilia does not correlate with CT scores.
Introduction
In the past few years, increasing evidence has emerged to support the central role of the eosinophil in the pathogenesis of chronic rhinosinusitis (CRS). It has long been known that eosinophilia is more prevalent in the mucosa of the patients with CRS than in control subjects [1,2]. Several recent studies have demonstrated the eosinophil to be the source of immunomodulators and cellular byproducts that influence the mucosal response to allergens and infection [3,4]. Therefore, the eosinophil may not only have a role in the pathogenesis of CRS but may also be a useful indicator of disease severity and prognosis for various forms of therapy.The aim of this study was to quantify peripheral eosinophilia and percentage of eosinophils in white blood cell (WBC) count of the patients with CRS and look for the correlation between the CT scores and peripheral eosinophilia.
Methods
The retrospective analysis of 60 consecutive patients who have medically refractory CRS was studied. There were 34 women and 26 men in the study group and the mean age of the patients was 34±21 years. All patients met clinical criteria established by The American Academy of Otolaryngology for the diagnosis of chronic sinusitis; thorough medical management had failed for all [5]. Patients with CRS were treated with amoxciline- clavunic acid 1 g 2 times a day for 2-4 weeks (depending on clinical observations) and mometazon furoat 50 mcg for each nostril topically, once a day for 12 weeks before radiological quantification. There were 12 patients (%20) with atopy in CRS group. Atopy was defined as one or more positive skin prick test to house dust mite, grass pollen, trees, weed, mould, cat and dog dander. Patients had coronal CT scans, and the extent of the disease was scored according to the classification of Lund and Mackay [6]. Normal anterior ethmoidal, posterior ethmoidal, maxillary, frontal and sphenoidal sinuses were assigned a score of 0. For mucosal thickening 1, and for total opacification 2 was assigned. The osteomeatal complex had a score of either 0 (normal) or 2 (mucosal thickening or obstruction). Each side was scored individually, and the total possible score was 12 (all together, for both sides, 24). The total CT score was used to determine whether patients had limited (1 to 11) or extensive (> 11) disease. All the patients in the study had CT scores greater than 6. Seventeen patients had extensive disease and nine of them were suffering from nasal polyposis. There were 60 people who had normal paranasal CTs (26 male and 34 female, 30±17 years old) for control group. There were no sinonasal sympthoms in the ones of control group. The axial CT was performed in control group for cranial trauma, or to determine the etiology of chronic headache. Peripheral eosinophil counts and the percentages of eosinophils in white blood cell count (WBC) were statistically compared with CT scores in CRS group. We also statistically analyzed the peripheral eosinophil counts and the percentages of eosinophils in WBC counts between CRS group and control group.Results
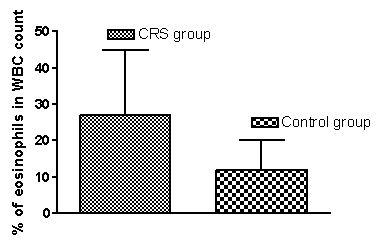
Eosinophil count was 3.22±2.06X103 and 1.49±0.95 X103 for CRS and control groups respectively. Percentage of eosinophils in white blood cell count was %27±18 and %12±8 for CRS and control group respectively. Mean CT score of CRS groups was 10.7±5.1. The values of peripheral eosinophil counts and the percentage of eosinophils in WBC count were demonstrated in figure 1, and figure 2 respectively. We performed independent samples t test for statistical analysis between CRS and control group. We found significant difference between CRS group and control groups for peripheral eosinophil count, and percentage of eosinophils in WBC count (p=0.001 and p=0.000 respectively).We also performed Pearson test for correlation analysis between CT scores and both peripheral eosinophil counts and percentages of eosinophils in CRS group. We observed no correlation between CT scores and both peripheral eosinophil counts, or percentages of eosinophils in WBC counts (p=0.067 and p=0.051) respectively.
 Büyütmek İçin Tıklayın |
Figure 1: Eosinophil counts for chronic rhinosinusitis and control groups (EC: eosinophil count) |
 Büyütmek İçin Tıklayın |
Figure 2: Percentage of eosinophils in white blood cell count for chronic rhinosinusitis and control groups. |
Discussion
The common denominator in CRS with or without nasal polyposis is the intense eosinophilic infiltration of the nasal mucosa, which is histological hallmark of the disease and is present independent from atopy [7]. According to the Ponikau et al [8] CRS would present an allergic immune reaction to fungus and so the function of the eosinophils would be to destroy fungal pathogens that were shown to be present in the mucine. In the majority of CRS cases, the eosinophils observed in the tissue were found to be merely in transit and, ultimately, to migrate into the mucin of the airway lumen. There, these eosinophils formed clusters, typifying the characteristic of eosinophilic mucin [8].Di Lorenzo et al recently found that blood eosinophil levels in patients with nasal polyps were significantly higher than those observed in control patients with allergic sinusitis but no polyps [9].We also found out that both blood eosinophil level and percentage of eosinophils in white blood cell count in patients with CRS were significantly higher than those observed in control group. The degree of peripheral eosinophilia has been shown to drop with a reduction in the load of nasal polyps treated either by surgical excision or by medical therapy [10]. Zadeh et al [11] found that those individuals with elevated serum eosinophilia counts suffered more postoperative sinus infections, required more subsequent sinus medications, and underwent more revisions sinus surgeries than control subjects.
It has been shown that the clinically inflamed sinus mucosa has an increase in cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF); interleukins 3, 4, and 13 (IL-3, IL-4, and IL-13); and the chemokine RANTES (regulated on activation normal T-cell expressed and secreted) that are involved in recruitment, proliferation, activation, and survival of eosinophils[12,13,14]. Immunohistochemical studies demonstrated activation of eosinophils is associated with subsequent degranulation and extra cellular deposition of toxic mediators such as eosinophil cationic protein (ECP) and major basic protein (MBP) that damage the epithelium and induce propagation of the inflammatory reaction [12,15,16]. It is known that systemic and local corticosteroid treatment caused a considerable decrease in the number of activated eosinophils and in serum ECP levels in patients with massive nasal polyposis [16]. Newman et al [17] examined the CT of patients with CRS and also reported that those with eosinophilia have a high likelihood of developing extensive disease. In recent study, analysis of the blood eosinophil count showed that it correlated with the degree of nasal polyposis [18].
Reference
1) Stoop AC, van der Heijden HA, Biewenga J, van der Baan S. Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol 1993;91:616-622 [ Özet ]
2) Bachert C, Van Cauwenberge PB. Inflammantory mechanisms in chronic sinusitis. Acta Otorhinolaryngol Belg 1997; 51(4):209-217 [ Özet ]
3) Besancon-Watelet C, Bene MC, Montagne P, Faure GC, Jankowski R. Eosinophilia and cell activation mediators in nasal secretion. Laryngoscope 2002; 112:43-46 [ Özet ].
4) Sobol SE, Christodolopoulos P, Manoukian JJ, Hauber HP, Frenkiel S, Desrosiers M, Fukakusa M, Schloss MD, Hamid Q. Cytokine profile of chronic sinusitis in patients with cystic fibrosis. Arch Otolaryngol Head Neck Surg 2002;128(11):1295-1298 [ Özet ]
5) Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997 117(3pt2):S1-7 [ Özet ].
6) Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinology 1993;31:183-184 [ Özet ].
7) Kaliner MA, Osguthorpe JD, Fireman P, Anon J, Georgitis J, Davis MC, Naclerro R, Kennedy D. Sinusitis: bench to bedside-current findings, future directions. Otolaryngol Head Neck Surg 1997;116(6pt2):S1-20 [ Özet ].
8) Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, Gaffey TA, Roberts GD. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc 1999;74(9):877-884 [ Özet ].
9) DiLorenzo G, Drago A, Esposito Pellitteri M, Candore G, Colombo A, Gervasi F, Pacor ML, Purella D’Ambrosio, Caruso C. Measurement of inflammantory mediators of mast cells and eosinophils in native nasal lavage fluid in nasal polyposis. Int Arch Allergy Immunol 2001;125:164-175 [ Özet ].
10) Metson R, Gliklich RE. Computed tomography to evaluate chronic sinusitis. JAMA 1994; 21:272(11);852 [ Özet ].
11) Zadeh MH, Banthia V, Anand VK, Huang C. Significance of eosinophilia in chronic rhinosinusitis. Am J Rhinol 2002;16:313-317 [ Özet ].
12) Hamilos DL, Leung DYM, Wood R, Meyers A, Stephens JK, Barkans J, Meng Q, Cunningham L, Bean DK, Kay AB. Chronic hyperplastic sinusitis: association of tissue eosinophilia with mRNA expression of granulocyte-macrophage colony-stimulated factor and interleukin-3. J Allergy Clin Immunol 1993;92(1pt1):39-48 [ Özet ].
13) Baroody FM, Hughes CA, McDowell P, Hruban R, Zinreich SJ, Naclerio RM. Eosinophilia in chronic childhood sinusitis. Arch Otolaryngol Head Neck Surg 1995;121(12):1396-1402 [ Özet ].
14) Al Ghamdi K, Ghaffar O, Small P, Frenkiel S, Hamid Q. IL-4 and IL-3 expression in chronic sinusitis: relationship with cellular infiltrate and effect of topical corticosteroid treatment. J Otolaryngol 1997;26:160-166 [ Özet ].
15) Harlin SL, Ansel DG, Lane SR, Myers J, Kephart GM, Gleich JA. A clinic and pathologic study of chronic sinusitis: the role of the eosinophil. J Allergy Clin Immunol 1988;81:867-875 [ Özet ].
16) Appenroth E, Gunkel AR, Müller H, Völklein C, Schrott-Fischer A. Activated and non activated eosinophils in patients with chronic rhinosinusitis. Acta Otolaryngol 1998;118(2):240-242 [ Özet ].
17) Newman LJ, Platts-Mills TA, Phillips CD, Hazen KC, Gross CW. Chronic sinusitis: a relationship of computed tomographic findings to allegy, asthma and eosinophilia. JAMA 1994;271:363-367 [ Özet ].
18) Bryson JM, Tasca RA, Rowe-Jones JM. Local and systemic eosinophilia in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis with and without polyposis. Clin Otolaryngol 2003; 28, 55-58 [ Özet ]




