OLFACTORY AND GUSTATORY DYSFUNCTION AND AUDIO-VESTIBULAR SYMPTOMS IN COVID-19 PATIENTS
2University of Health Sciences, Kayseri City Training and Research Hospital, Audiology and Speech Pathology Department, Kayseri, Turkey
3Ege University Medical Faculty, Department of Physical Medicine and Rehabilitation, Izmir, Turkey
4University of Health Sciences, Kayseri City Training and Research Hospital, Department of Otorhinolaryngology and Head and Neck Surgery, Kayseri, Turkey
Summary
Objective: The study aims to determine subjective audio-vestibular symptoms with questionnaires and olfactory and gustatory dysfunction with visual analog scales among the inpatient and outpatient COVID-19 patients in accordance with their receiving the diagnosis period, and to compare the results.Materials and Methods: This two-centered study consist of 150 COVID-19 patients. The patients are categorized into three groups in accordance with their receiving the diagnosis period (14-30 days, 31-90 days, and 91-270 days) and each group consists of two sub-groups in terms of the setting of treatment (inpatient and outpatient). The following questionnaires and scales have been administered to the study group: Tinnitus Handicap Inventory (THI), Amsterdam Inventory for Auditory Disability and Handicap (AIADH), Vestibular Disorders Activities of Daily Living Scale (VADL), Visual Analogue Scale (VAS) - dizziness severity degree, Visual Analogue Scale (VAS) - smell and taste loss severity degree.
Results: The Visual Analogue Scale (VAS) - dizziness severity degree appeared to be significantly higher in the groups with receiving the diagnosis period 14-30 days and 31-90 days in comparison to the group with the receiving the diagnosis period 91-270 days. Further, in both two groups, VADL scores of the inpatient patients appeared to be significantly higher in comparison to outpatients" scores of the above-mentioned questionnaire. The total amount of patients who regained their smell and taste senses in the group with receiving the diagnosis period of 14-30 days is significantly lower in comparison to the other two groups. There is no statistically significant difference determined between groups in terms of the other questionnaires and scales.
Conclusion: As the severity of the infection increases in the first months after the diagnosis of COVID-19, COVID-19 has harmful effects, especially on balance and olfactory and gustatory functions, but recovery in these symptoms of patients is observed in the following period.
Introduction
The Novel Coronavirus Disease (COVID-19) pandemic started in December 2019 in the city of Wuhan, province of Hubei, People's Republic of China. The virus is a highly contagious zoonosis produced by the beta coronavirus (SARS-CoV-2), which is defined to have the transmission ability of human-to-human through the respiratory route. Within a few weeks, the disease caused by the virus had spread to Asian countries, Europe, America, and finally the whole world, demonstrating a rapid and asymptomatic but highly contagious prodrome[1]. Symptoms and tissue tropism of Coronavirus infection range across different host species. Coronavirus infections in terms of humans as the host species might be asymptomatic or accompanied by fever, cough, shortness of breath, and gastrointestinal irritation. In certain cases, especially in the group of elderly and immunocompromised individuals, Coronavirus infections can cause severe pneumonia and subsequent death of the patient[2]. The audio-vestibular symptoms associated with COVID-19 are determined as smell and taste loss, hearing loss, tinnitus, dizziness, and vertigo[3].An experimental study conducted on the SARS-CoV demonstrated a transneural penetration from the olfactory bulb[4]. Korkmaz et al. evaluated the otolaryngology symptoms of in-patient COVID-19 patients and revealed that there were symptoms associated with taste and smell senses. Further, Korkmaz et al. reported the hypogeusia (decrease in taste sense) in 41.3%, hyposmia/anosmia (decrease in smell sense) in 37.9%, dizziness in 31.8%, tinnitus in 11.2%, hearing loss in 5.2%, and vertigo in 6.1% in the study group of the total number of 116 COVID-19 patients[5]. İnan et al. found that 37% of COVID-19 patients had anosmia and 29% had hyposmia[6].
Within the systematic review study conducted by Almufarrij and Munro, it was emphasized that there are few cross-sectional and case studies regarding the effect of COVID-19 on the audio-vestibular system[3]. The studies suggest that evaluation was made subjectively utilizing the medical records of the patients or self-reported scales. In their systematic review study, solely four of the studies utilized an audiological test battery and a study utilized a validated hearing-specific quality of life scale[7-10]. In the literature, Tan et al. evaluated COVID-19 patients" audio-vestibular symptoms with objective test batteries and found that COVID-19 patients had pathological VNG head shake values, higher threshold results in pure tone audiometry results, lower gain in vHIT and asymmetric findings in oVEMP and cVEMP tests compared to control group[11]. Gedik et al. found that COVID-19 patients" interpeak latencies of waves III-V in ABR test were significantly more different and their TEAOEs were bilaterally lower at 4 kHz than control group[12].
In addition, studies in the literature generally evaluated otological and audiological symptoms of patients in the first three months after the diagnosis[5,11,13-19]. These studies compare the severity of the disease (from mild to serious and critical level). A limited number of studies compare the symptoms of patients in the acute phase of COVID-19 and progressing symptoms of 6-7 months[20-22]. Conversely, few studies compare the symptoms of COVID-19 in patients with regard to in-patient and out-patient treatments[17,23].
Within this context, the aims of this study are to determine subjective audio-vestibular symptoms and olfactory and gustatory dysfunction with questionnaires and visual analog scales among the inpatient and outpatient COVID-19 patients in accordance with their receiving the diagnosis period (14-30, 31-90 and 91-270 days), and to compare the results with questionnaires and scales.
Methods
This cross-sectional and two-centered study was carried out between February 25, 2021, and June 01, 2021, in the ENT clinic and physical therapy and rehabilitation clinic which was converted into a COVID-19 clinic. The study has the approval of the Ministry of Health (2021-01-24T19_51_36) and the approval of the clinical ethics committee of Kayseri City Hospital (12.02.2021/47). The data of the study group who have been diagnosed with COVID-19 was obtained from the data recording system of two clinics and the study group was informed regarding the study via phone. The study group was selected with the sampling method based on volunteerism which is one of the non-probability sampling methods. Face-to-face questionnaires were utilized in the study and the study group was invited to the clinics by the researchers to provide data for scales. The study group granted written consent with an informed consent form. The study group consists of 150 adult participants (92 women and 58 men) from two above-mentioned clinics. The sample of this study was determined by power analysis using the G*power 3.1 program.Participants who have a positive PCR test via nasopharyngeal and oropharyngeal swabs and are diagnosed with COVID-19 once; have no hearing, balance, tinnitus, and smell and taste loss issues before being diagnosed with COVID-19 and have no chronic disease; have not been treated for COVID-19 in the intensive care unit; do not use any medical drugs regularly; do not have any ear-nose-throat operations in the past and who are between the ages of 18-45 constitute the study group.
The participants of the study group have been categorized into three sub-sections in terms of receiving the diagnosis period as follows: 14-30 days, 31-90 days, and 91-270 days. Further, each section has been categorized into two sections in terms of treatment setting as follows: inpatient or outpatient. Whilst the patients who were receiving the treatment outpatient experienced COVID-19 symptoms as in a mild state, the patients who were receiving the treatment inpatient experienced COVID-19 symptoms as in a serious state. In terms of determining the severity degree of the symptoms, the study refers to and utilizes "National Interim Guidance and World Health Organization (WHO) Interim Clinical Management Guidance"[24,25].
The following scales and questionnaires were administered to the study group: Demographic Information Form (including age, gender, date of The diagnosis of COVID-19, Setting of Treatment [At-home (outpatient) or At-hospital (inpatient)], COVID-19 Symptoms), Tinnitus Handicap Inventory (THI), Amsterdam Inventory for Auditory Disability and Handicap (AIADH), Vestibular Disorders Activities of Daily Living Scale (VADL), Visual Analogue Scale (VAS) - dizziness severity degree, Visual Analogue Scale (VAS) - smell and taste loss severity degree.
Tinnitus Handicap Inventory (THI)
Tinnitus handicap inventory (THI) refers to a questionnaire that consists of 25 questions. Aksoy et al. translated the mentioned questionnaire into Turkish in 2006 and conducted its Turkish validity and reliability[26].
Amsterdam Inventory for Auditory Disability and Handicap (AIADH)
Kramer et al. who evaluated the individuals" level of auditory disability and the perception of disability related to it developed this scale in 1995[27]. Mujdeci et al. conducted the scale's Turkish validity and reliability[28].
Vestibular Disorders Activities of Daily Living Scale (VADL)
The Vestibular Disorders Activities of Daily Living Scale aims to evaluate the effect of vestibular symptoms experienced by patients on activities on daily basis. Cicek Cinar et al. conducted the scale's Turkish validity and reliability[29].
Visual Analogue Scale (VAS) - Dizziness Severity Degree
The participants rate the severity of dizziness between 0 (no dizziness) and 10 (very severe dizziness).
Visual Analogue Scale (VAS) - Smell and Taste Loss Severity Degree
The scale requests participants to rate the severity of smell and taste loss between 0 (very severe loss of smell and taste) and 10 (normal sense of smell and taste). The scale also consists of the part where participants are requested to state for how many days the symptoms of loss of smell and/or taste had lasted.
Statistical Analysis
The analysis of the data of the research was executed with the SPSS (Statistical Program in Social Sciences) 25 Program. The study data's convenience to the normal distribution was affirmed with the Kolmogorov Smirnov Test. The statistically significance level (p) for comparison tests is appointed as 0.05.
Variance homogeneity has been checked with the Levene Test. Two-groups pairs" comparison phase was conducted with Two Independent Samples T Test and multiple-groups comparison phase were conducted with ANOVA test. The study utilizes Tukey's multiple comparison (post-hoc) test to determine the groups with a difference which appears to be a result demonstrated by the ANOVA test.
The study performs χ2 analysis by creating crosstabs for categorical data.
Results
Demographic InformationThe variance of the study group's 150 participants information regarding their sex and setting of treatment (outpatient or inpatient) to their receiving the diagnosis period of COVID-19 (14-30 days, 31-90 days, 91-270 days) are calculated as in percentages and numeric data and the results are given in Table 1.
Table 1: The variance of Demographic Variables by Groups
The average age of the participants of the study is identified as 33.62 ± 7.83, and there was no statistically significant difference between the groups.
Whilst the number of participants who received their treatment inpatient was 72 (48%), the number of participants who received their treatment outpatient was 78 (52%). According to the data, the average staying at hospital period of the participants of the study appears to be 4.76 ± 3.36 days.
Figure 1 demonstrates the percentage distribution and the total number of audio-vestibular, smell and taste loss, and other ENT symptoms of all participants related to COVID-19. The most common symptoms are determined as loss of smell (58.1%), loss of taste (55.4%), and dizziness (27.7%).
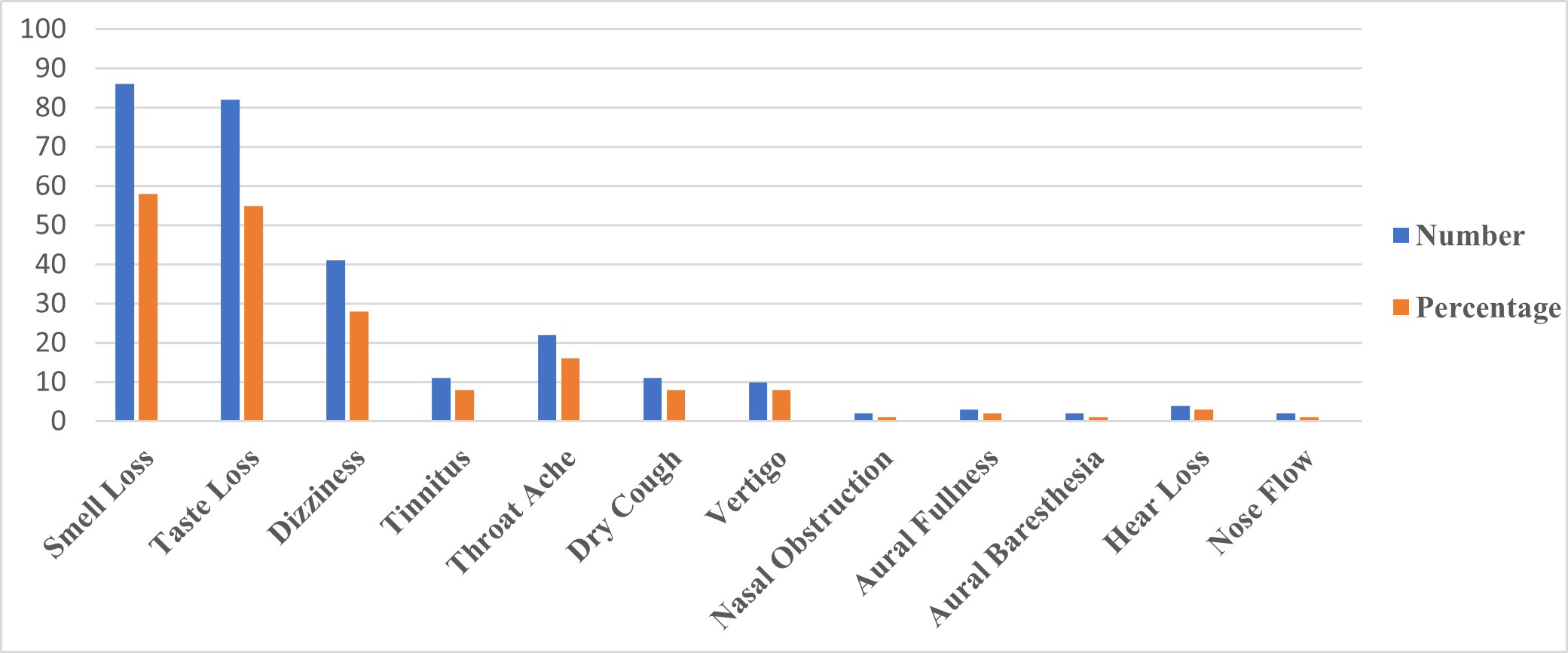 Büyütmek İçin Tıklayın |
Figure 1: The percentage distribution and the total number of audio-vestibular, smell and taste loss, and other ENT symptoms of all participants related to COVID-19. |
Comparison of Test Results of Study Groups
A statistically significant difference is not observed between the groups constituted in accordance with the receiving the diagnosis period of the study's participants (14-30 days, 31-90 days, 91 days and above) in terms of THI, VADL, AIADH, (VAS) - Smell Loss Severity Degree, (VAS) - Taste Loss Severity Degree (p>0,05).
A statistically significant difference is observed between the groups' VAS - Dizziness Severity Degree scores (p<0.05). Since there was no homogeneity of variance (p>0.05) when the testing phase was being conducted to determine whether there was a difference between the groups, Tamhane T2 post-hoc test was used. The Test results are given in the table 2.
Table 2. Paired Comparisons for the Variable with Statistically Significant Difference
In terms of the VAS - Dizziness Severity Degree Scale variable, the current VAS - Dizziness Severity Degree Scale scores (0.08 ± 0.45, respectively) of the groups with receiving the diagnosis of COVID-19 period of 14-30 days ago and 31-90 days ago are identified as to be statistically significantly higher in comparison to the group with receiving the diagnosis period of 91-270 days ago (p<0.05, Table 2).
In order to realize whether there is a difference between the groups in terms of recovery time of the taste and smell senses of the participants in the three groups, a crosstab is created and the χ2 test statistic was calculated. The results are given in Table 3.
Table 3: Comparison of Groups in Terms of Recovery Time of the Taste and Smell Senses
A statistically significant difference is observed between the regaining of taste and smell senses and the duration of receiving the diagnosis of COVID-19 period in the groups (14-30 days, 31-90 days, 91-270 days) (p<0.05, Table 3). In the group with receiving the diagnosis of 14-30 days period, the number of those who regained their smell and taste senses appears to be significantly lower than the number of those in the other two groups (p<0.05).
Study groups have regained their smell sense completely in the following periods: 19 patients in the group with receiving the diagnosis period of 14-30 days regained their smell sense in 14,21 ± 9,32 days, 31 patients in the group with receiving the diagnosis period of 31-90 days regained their smell sense in 14,65 ± 13,8 days and 32 patients in the group of receiving the diagnosis period of 91-270 days regained their smell sense in 24,72 ± 23,89 days.
Twenty patients in the group with receiving the diagnosis period of 14-30 days, 32 patients in the group with receiving the diagnosis period of 31-90 days, and 29 patients in the group with receiving the diagnosis period of 91-270 days regained their taste sense completely.
Comparison of the Effect of the Setting of Treatment Variable on the Test Results Among the Groups
In the group of receiving the diagnosis period of 14-30 days, a statistically significant difference is not observed in terms of the scores of THI, VADL-Total, VADL-Functional, VADL-Instrumental, AIADH, VAS - Dizziness Severity Degree, VAS - Smell Severity Degree, VAS -Taste Severity Degree between inpatient and outpatient participants (p>0,05). However, in this group, the VADL-Ambulation score of the at-hospital participants appears to be statistically significantly higher than the scores of those who received the treatment outpatient (p<0.05).
In the group with receiving the diagnosis period of 31-90 days, a statistically significant difference is not observed in terms of the scores of THI, VADL-Total, VADL-Functional, VADL-Instrumental, AIADH, VAS - Dizziness Severity Degree, VAS - Smell Severity Degree, VAS - Taste Severity Degree between inpatient and outpatient participants (p>0,05). However, in this group, the VADL-Ambulation score of the inpatient participants appears to be statistically significantly higher than the scores of those treated outpatient (p>0.05). In addition, VADL-total, VADL-functional, and VADL-ambulation scores of the inpatient patients statistically appear to be at a statistically significantly higher level in comparison to the same scores of the outpatient patients (p<0,05).
In the group with receiving the diagnosis period of 91-270 days, a statistically significant difference is not observed in terms of the scores of THI, VADL-Total, VADL-Functional, VADL-Instrumental, AIADH, VAS - Dizziness Severity Degree, VAS - Smell Severity Degree, VAS - Taste Severity Degree, VADL-total, VADL-functional, and VADL-ambulation score between inpatient and outpatient participants (p>0,05).
There is no observed statistically significant difference in the groups of different receiving the diagnosis periods between the variable of the setting of treatment (outpatient and inpatient) and the regaining of taste and smell senses (p>0.05).
Discussion
The virus enters the body and stays in oropharyngeal and nasopharyngeal mucosa, symptoms related to the ear, nose, and throat are common in COVID-19[14]. The study revealed that the most common symptoms related to COVID-19 in the participants of the study group are loss of smell (58.1%) and loss of taste (55.4%). In a similar study which included 180 COVID-19 patients conducted by Savtale et al., it is reported that 100 patients experienced the smell loss (55.5%) and 106 patients experienced the taste loss (58.88%)[30]. In a systematic review study consists of 24 studies including 8438 COVID-19 patients" data, 41.0% of olfactory dysfunction rate and 38.2% of taste dysfunction rate were observed. In accordance with this study, it was highly emphasized that a high prevalence of olfactory and gustatory dysfunction among COVID-19 patients is present[31]. The angiotensin-converting enzyme 2 receptor, utilized by SARS-CoV-2 to bind, diffuse, and enter cells is widely exposed in epithelial cells of the oral cavity mucosa. Therefore, they stated that these findings might be the bioinformatic evidence to explain the potential infection routes of the underlying taste and smell disorders in SARS-CoV-2 infection and highly associated with accompanying symptoms[32]. In our study, most of the participants regained their sense of taste and smell in first 30 days. No significant difference was observed between the VAS scores of the groups in accordance with the duration of receiving the diagnosis period of COVID-19 (14-30 days, 31-90 days, 91-270 days) in terms of the smell and taste loss severity degree. Further, there was no statistically significant difference between the groups in terms of the setting of treatment (inpatient or outpatient). Nonetheless, when two groups are compared, the number of those who regained their smell and taste senses was observed to be statistically significantly lower in the group with the receiving the diagnosis period of 14-30 days. On the other hand, D'ascanio et al. found that outpatients reported loss of smell more than inpatients[33]. They obtained that all patients recovered by 30 days. In the study of Salepci et al., they revealed that the smell and taste loss was still present in 6 patients out of 223 patients at the end of one month[14]. Since the study of Salepci et al. is a study investigating short-term COVID-19 ENT symptoms, authors emphasize that there is no possible and certain way to assure how long the symptoms will last or whether they will be permanent. Our study comprises the period of 270 days after receiving the diagnosis of COVID-19. There is only one participant who did not regain taste sense in the group with the receiving the diagnosis period of 91-270 days and every participant regained smell sense. Moreover, each participant regained smell and taste senses completely at the end of one month. Stavem et al. reported that among 451 patients, only 12% of patients experienced smell loss and 10% of patients cannot regain their taste sense 1.5-6 months after receiving the diagnosis of COVID-19[20]. Consequently, Stavem et al. considered and claimed that recovery in symptoms primarily occurs in the first few weeks after the acute phase of the disease. In the light of the results and findings retrieved from our study and the literature, it is revealed that the smell and taste loss can be recovered in a short time and regained almost completely, including patients who experienced COVID-19 in a highly early period and received their treatment at-hospital with critical symptoms.Our study reveals that the third most common symptom related to COVID-19 experienced by the participants of study groups is dizziness (27.7%). Groups with receiving the diagnosis of COVID-19 period of 14-30 and 31-90 days experienced statistically significantly higher dizziness symptoms labeled in VAS dizziness severity degree than the group with receiving the diagnosis period of 91-270 days. In terms of the VADL scale scores, validated and reliable in the Turkish Medical Framework, there is no statistically significant difference between the groups. Nevertheless, in the VADL subscales, which evaluate functional and ambulation skills and are especially related to activities requiring walking or position change of body, inpatient patients of these two groups had a statistically significantly higher score than outpatient patients. The scores of those receiving the diagnosis period of at least 91 days ago did not differ in line with whether they were inpatient or outpatient patients. In our study, inpatient participants experienced serious symptoms of COVID-19. The data demonstrates that especially in the first 90 days after receiving the diagnosis of COVID-19, serious COVID-19 symptoms can affect the vestibular systems of the patients in the acute and sub-acute period, and these patients might recover after three months. Tan et al. emphasized that due to extensive hypercoagulation, SARS-CoV-2 may cause direct neurological or inner ear involvement[11]. Within the study of Goërtz et al., they revealed that there is no statistically significant difference was observed between the dizziness in patients who were receiving their treatment at-hospital and those who were receiving their treatment at-home during the infection. In addition, the prevalence of dizziness syndrome decreased from 52% to 27% in an average follow-up period of 79 days[17].
Viola et al. reported that in the first 30-60 days after receiving the diagnosis of COVID-19, 18.4% of the patients experienced dizziness and 23.2% of them experienced tinnitus[18]. In our study, the prevalence of patients who experienced tinnitus was 7.4%, and the prevalence of the patients who experienced hearing loss was 2.7%. AIADH and THI results of the study did not differ between groups. In another study related to the subject, 50 patients who were tested negative in SARS-COV-2 RT-PCR and administered the Hearing Handicap Inventory for Adults (HHIA) test after 15 days of PCR test, the results demonstrate that hearing loss worsened in 9 (18%) of total 50 patients. According to the THI result, it was reported that five (10%) of the total 50 patients experienced mild tinnitus. It was revealed that that the average score measured by THI in these patients demonstrated little or no perceived activity limitations or participation restrictions[10]. In another study, as the results of the pure tone audiometry test and transient otoacoustic emission test of the asymptomatic COVID-19 patients" group, significantly worse hearing thresholds and lower amplitude instantaneous evoked otoacoustic emission were obtained at high frequencies[8]. Gedik et al. also found similar objective test results[12]. Our study and the results retrieved from these studies demonstrate that COVID-19 infection, regardless of symptoms" severity and duration, may have detrimental effects on cochlear cell functions from early diagnosis and recovery therein is also present in long term.
It has been suggested that different coronavirus types, including SARS-COV-2, might enter the body through peripheral nerves using intranasal grafting or trans-synaptic pathways, thereby infecting both neurons and neuroglia. Therefore, the mechanisms of damage to the peripheral auditory system cause direct viral damage to the corti organ, the stria vascularis, or spiral ganglion. It includes damage mediated by the patient's immune system against virally expressed proteins (Cytomegalovirus) and immunodeficiency leading to secondary bacterial infection in the ear[34]. Further, it is revealed that SARS-CoV-2 may cause vascular damage, which is caused by vasculitis or vasculopathy. It is claimed that hearing alterations and balance disorders may develop as a result of vascular damage, since inner ear structures may be particularly susceptible to ischemia due to terminal vasculature characteristics and high energy requirement[18]. These assumptions suggest that COVID-19 infection might have a relation with audio-vestibular findings in the literature. Nonetheless, in the systematic review article of Salari et al., they stated that the prevalence of stress, anxiety, and depression in the population was high during the pandemic period[35]. Therefore, as the authors, we consider that emotional distress such as anxiety and depression might be a factor that can increase tinnitus and dizziness.
Our study compares the audio-vestibular and smell and taste loss symptoms related to the diagnosis of COVID-19 with the validated and reliable subjective scales in accordance with the acute, sub-acute, and long-term and at-hospital or at-home treatment status. Further, in contrast to many studies in the field, patients with these symptoms prior to COVID-19 infection were excluded from the study, considering that this could demonstrate much clearly the impact of COVID-19 on audio-vestibular system symptoms and olfactory and gustatory dysfunction. In order to exclude the possibility of presbycusis and old age-related central vertigo, the age range of the study was limited to 18-45 years. Nevertheless, our study includes certain limitations such as objective measurement methods for the evaluation of smell-taste function, hearing and vestibular system were not utilized. In addition, since the psychological factors of the patients were not evaluated, the impact of these factors on the findings is unknown. For the studies that will be conducted in future, it is considered that including more patients, utilizing objective test methods as well as subjective test methods in evaluation, and investigating the impact of psychological factors shall contribute to the literature highly.
Conflicts of interest/competing interests: All authors declare that they have no conflict of interest.
Acknowledgements
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Reference
1) Kowalski LP, Sanabria A, Ridge JA, Ng WT, de Bree R, Rinaldo A, Takes RP, Mäkitie AA, Carvalho AL, Bradford CR, Paleri V, Hartl D M, Vander Poorten V, Nixon IJ, Piazza C, Lacy PD, Rodrigo JP, Guntinas-Lichius O, Mendenhal WM, D'Cruz A, Lee AWM, Ferlito A. COVID-19 pandemic: Effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head neck 2020; 42:1259-67. [ Özet ]
2) Sharma A, Ahmad Farouk I, Lal SK. COVID-19: A review on the novel coronavirus disease evolution, transmission, detection, control and prevention. Viruses 2021; 13:202. [ Özet ]
3) Almufarrij I, Munro KJ. One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol 2021; 60:935-945. [ Özet ]
4) Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008; 82:7264-75. [ Özet ]
5) Özçelik Korkmaz M, Eğilmez OK, Özçelik MA, Güven M. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Euro Arch Oto-Rhino-L 2021; 278:1675-85. [ Özet ]
6) İnan S, Özer F, Erbek S, Çaylaklı F, Ödemiş İ, Kurşun E. Olfactory disorders in patients with mild to moderate COVID-19: spontaneous recovery in one-month follow up. B ENT 2021; 17:18-23.
7) Dror AA, Kassis-Karayanni N, Oved A, Daoud A, Eisenbach N, Mizrachi M, Rayan D, Francis S, Layous E, Gutkovich YE, Taiber S, Srouji S, Chordekar S, Goldenstein S, Ziv Y, Ronen O, Gruber M, Avraham KB, Sela E. Auditory Performance in Recovered SARS-COV-2 Patients. Otol Neurotol 2021; 42:666-70. [ Özet ]
8) Mustafa MWM. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol 2020; 41:102483. [ Özet ]
9) Gallus R, Melis A, Rizzo D, Piras A, De Luca LM, Tramaloni P, Serra A, Longoni E, Soro GM, Bussu F. Audiovestibular symptoms and sequelae in COVID-19 patients. J Ves Res 2021; 31:381-387. [ Özet ]
10) Freni F, Meduri A, Gazia F, Nicastro V, Galletti C, Aragona P, Galletti C, Galletti B, Galletti F. Symptomatology in head and neck district in coronavirus disease (COVID-19): A possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol 2020; 41:102612. [ Özet ]
11) Tan M, Cengiz DU, Demir İ, Demirel S, Çolak SC, Karakaş O, Bayındır T. Effects of Covid-19 on the audio-vestibular system. Am J Otolaryngol 2022; 43:103173. [ Özet ]
12) Gedik Ö, Hüsam H, Başöz M, Tas N, Aksoy F. The effect of coronavirus disease 2019 on the hearing system. J Laryngol Otol 2021; 135:810-4. [ Özet ]
13) Carfì A, Bernabei R, Landi F. Persistent Symptoms in Patients After Acute COVID-19. Jama 2020; 324:603-5. [ Özet ]
14) Salepci E, Turk B, Ozcan SN, Bektas ME, Aybal A, Dokmetas I, Turgut S. Symptomatology of COVID-19 from the otorhinolaryngology perspective. Euro Arch Oto-Rhino-L 2021; 278:525-35. [ Özet ]
15) Elibol E. Otolaryngological symptoms in COVID-19. Euro Arch Oto-Rhino-L 2021; 278:1233-6. [ Özet ]
16) Munro KJ, Uus K, Almufarrij I, Chaudhuri N, Yioe V. Persistent self-reported changes in hearing and tinnitus in post-hospitalisation COVID-19 cases. Int J Audiol 2020; 59:889-90. [ Özet ]
17) Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, Houben-Wilke S, Burtin C, Posthuma R, Franssen FME, van Loon N, Hajian B, Spies Y, Vijlbrief H, van 't Hul AJ, Janssen DJA, Spruit MA. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ open res 2020; 6: 00542-2020. [ Özet ]
18) Viola P, Ralli M, Pisani D, Malanga D, Sculco D, Messina L, Laria C, Aragona T, Leopardi G, Ursini F, Scarpa A, Topazio D, Cama A, Vespertini V, Quintieri F, Cosco L, Cunsolo EM, Chiarella G. Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Euro Arch Oto-Rhino-L 2021; 278: 3725-3730. [ Özet ]
19) Hassani S, Lazem M, Jafari Z. No lasting impact of Covid-19 on the auditory system: A prospective cohort study. J Laryngol Otol 2021:1-6.
20) Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2020; 76:405-7. [ Özet ]
21) Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, Redfield S, Austin JP, Akrami A. Characterizing long covid in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38:1-19. [ Özet ]
22) Petrocelli M, Cutrupi S, Salzano G, Maglitto F, Salzano FA, Lechien JR, Saussez S, Boscolo-Rizzo P, De Riu G, Vaira LA. Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study. J Laryngol Otol 2021; 135:436-41. [ Özet ]
23) Körkuyu Yardımcı E, Paksoy Zehra Betül. Covid-19 hastalarında koku ve tat kaybı. Kbb Forum 2023; 22:122-129.
24) TMoHS B. COVID-19 (SARS-CoV-2 Infection) Guide 2020. https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID-19_Rehberi20200414_eng_v4_002_14.05.2020.pdf. 2020.(Erişim Tarihi: 01/05/2021)
25) WHO O. Clinical management of COVID-19 2020. https://www.who.int/publications/i/item/clinicalmanagement-of-covid-19. 2020. (Erişim Tarihi: 01/05/2021)
26) Aksoy S, Firat Y, Alpar R. The Tinnitus Handicap Inventory: a study of validity and reliability. Int Tin J 2007; 13:94-8. [ Özet ]
27) Kramer SE, Kapteyn TS, Festen JM, Tobi H. The relationships between self-reported hearing disability and measures of auditory disability. Audiol 1996; 35:277-87. [ Özet ]
28) Mujdeci B, Inal O, Turkyilmaz M, Kose K. Turkish translation, reliability and validity of the amsterdam inventory for auditory disability and handicap. J Indian Speech Hearing Assoc 2016; 30:40-6.
29) Çiçek Çınar B, Kaya Ş, Pektaş Sjöstrand A, Alpar R, Aksoy S. Turkish validity and reliability of vestibular disorders activities of daily life. Fiz Rehabil 2017; 28:1-11.
30) Savtale S, Hippargekar P, Bhise S, Kothule S. Prevalence of Otorhinolaryngological Symptoms in Covid 19 Patients. Indian J Otolaryngol Head Neck Surg 2022; 74:3378-3384. [ Özet ]
31) Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin Proc 2020; 95:1621-31. [ Özet ]
32) Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020; 12:8. [ Özet ]
33) D'Ascanio L, Pandolfini M, Cingolani C, Latini G, Gradoni P, Capalbo M, Frausini G, Maranzano M, Brenner MJ, Di Stadio A. Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol Head Neck Surg 2021; 164:82-6. [ Özet ]
34) Abramovich S, Prasher DK. Electrocochleography and brain-stem potentials in Ramsay Hunt syndrome. Arch Otolaryngol Head Neck Surg 1986; 112:925-8. [ Özet ]
35) Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, Rasoulpoor S, Khaledi-Paveh B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob Health 2020; 16:57. [ Özet ]




