THE EFFECT OF L-ORNITHINE ON THE INNER EAR OF RATS IN THE LIPOPOLYSACCHARIDE INDUCED ENDOTOXEMIA MODEL
2Department of Pathology, University of Mersin, Mersin, Turkey
3Department of Pharmacology, University of Mersin, Mersin, Turkey
4Department of Biochemistry, University of Mersin, Mersin, Turkey
5Department of Anesthesiology and Reanimation, University of Mersin, Mersin, Turkey
Summary
Objectives; To investigate the relationship between ornithine treatment, p53 expression and the histopathologic differentials of rats inner ear in endotoxemia induced by lipopolysaccharide.Material and methods; Twentyfour healthy rats were randomly divided into 4 groups. The first group, served as control, received intraperitoneal saline; the second group, intraperitoneal endotoxin; the third group, intraperitoneal ornithine and saline, and the fourth group received intraperitoneal ornithine ten minutes before endotoxin administration.
Results; Congestion and subepitelial edema were observed in stria vascularis, and leukocyte, plasma cell infiltration in the parts of membraneuos labyrinthitis of modiolar region in the cochlea of the second and the fourth group. Inflammation was not detected in the parts of vestibulocochlear and facial nerves in meatus acousticus internus. When we compared the plasma levels of nitrite/nitrate and MDA among groups, we found statistically significant differences: between groups 1 and 2, 1 and 3, 1 and 4, 2 and 3, 3 and 4. However, no significant difference was found in groups 2 and 4. Ornithine did not reduce the harmful effect of sepsis in rats. Immunohistochemically, p53 immunoreactive cells were not detected in any groups.
Conclusion; L-ornithine can not improve the manifestations of septic picture in the rat cochlea.
Introduction
The prime initiatiator of gram-negative bacterial sepsis is endotoxin, a lipopolysaccharide (LPS) component of the bacterial outer membrane, which induces strong inflammation and cell infiltrations in the cochlea [1,2]. It has been shown that cytokines, released during endotoxemia, can induce nitric oxide synthase (iNOS) in cells which in turn causes production of nitric oxide (NO) in great amounts [3,4]. NO has been demonstrated as an inducer of apoptosis for several years [5,6]. During NO mediated apoptosis, as a tumor suppressor gene product, p53 has been shown to accumulate in macrophages [7]. Additionally, numerous noxious stimuli such as oncogen activation, hypoxia, hiperoxia, temperature changes, inflamation and DNA damage activate p53 via different mechanism . The activation of p53 due to overproduction of NO during LPS-induced endotoxemia increases cell apoptosis in parenchymall tissues [8]. Consequently, their functions may be disconcerted. The aim of therapeutic strategies is to inhibit iNOS or scavenging NO radicals with chemical moieties [1]. It was reported that L- ornithine prevented overproduction of NO to inhibit transport of L-arginin [9]. In order to detect the levels of NO radicals indirectly and oxidative states of organism, the blood levels of malondialdehyde (MDA), nitrate and nitrite may be measured. The lower blood levels of MDA, nitrite and nitrate indicate the sufficient antioxidant effect of L-ornithine via decreasing overproduction of NO. In sepsis due to the overproduction of NO from L-arginine, there is an extra demand of this amino acid, which is carried by the same transport system that can be inhibited by L-ornithine [1,9]. Therefore, we hypothesized that if this transporter was blocked by ornithine, the massive formation of NO could be prevented in sepsis. In the present study, we aimed to investigate the relationship between ornithine treatment, p53 expression and the histopathologic changes in the inner ear of rats in endotoxemia induced by lipopolysaccharide.Methods
The experiments described in this article were performed in adherence to institutional animal care and study guidelines on the use of experimental animals. This experimental protocol was approved by the ethic committee of our institute. Twentyfour healthy male Wistar rats, weighing 220 300 g, were housed at constant temperature with 14/10 h periods of light and dark exposure, respectively.
Experimental protocol:
The rats were randomly divided into 4 groups. The first group, served as control, received intraperitoneal saline (1ml/kg, n=6); the second group, received intraperitoneal endotoxin (Escherchia coli lipopolysacchardie 055: B5 10 mg kg-1 Sigma St Louis, Missouri, n=6); the third group, intraperitoneal saline and L- ornithine (500 mg kg-1, O2375, Sigma, St Louis, Missouri, n=6), and the fourth group received intraperitoneal L- ornithine (500 mg kg-1) 10 minutes before endotoxin administration (n=6).
Histopathology
Six hours later, rats were anaesthetized with intramuscular ketamine 50 mg kg-1 and xylazine 7 mg kg-1 and animals were sacrificed. The temporal bones were dissected and immersed in the fixative at 4 °C overnight. After decalcification with 10 % ethylenediaminetetraacetic acid, they were processed for paraffin embedding. Sections (4 mm) were cut at 40 mm intervals (midmodiolar hemisection) and stained with hematoxylin and eosin. To assess the severity of inflammation on inner ear cells, the number of inflammatory cells, including polymorphonuclear (PMN) cells, lymphocytes and macrophages were counted (Olympus Bx 50, Japan).
Immunohistochemical staining procedure
The distribution of p53 positive cells were examined by the avidinbiotin-peroxidase immunohistochemical method. Sections were cut in 5 m thickness and placed onto poly-L-lysine-coated slides. They were dried at room temperature overnight. Following this, sections were deparaffinized in xylene through ethanol to phosphatebuffered saline (PBS; pH 7.2). Endogen peroxidase activity was inhibited using 0.3 % solution of H2O2 in pH 7.5 PBS. Next, the sections were incubated 2 hours with p53 mouse antihuman monoclonal kit (clone DO-7, DAKO, 1:75). Following the first incubation, the sections were incubated for 30 minutes in avidin-biotin-complex peroxidase kit (Labvision Corporation, Fremont, USA). Finally the sections were counter-stained with Mayer's hematoxylen. The number and localization of p53 immunoreactive cells were evaluated in the cochlea.
Detection of plasma nitrate and malondialdehyde levels :
Blood samples were collected from each rat by cardiac puncture. We detected NO via nitrate which presents in the sample. Nitrate was reduced to nitrite by nitrate reductase and the formed nitrite was put to react with sulpanilamide and N-(1-naphtyl)-ethylenediamine to give a red-violet diazo dye. The diazo dye was measured on the basis of its absorbance in the visible range at 550 nm (Nitric Oxide Colormetric Assay, Boehringer Manheim, Cat. No. 1756281, Mannheim, Germany).
The levels of serum malondialdehyde (MDA) were measured spectrophotometrically by the method described by Yagi [11]. The final results were expressed as nmol of MDA formed per milliliter of serum. The differences between four groups were evaluated by t-test and p< 0.05 was accepted as significant.
Results
HistopathologyCongestion and subepitelial edema were observed in stria vascularis, and also in filtration of leukocyte and plasma cell infiltration in perimodiolar region of membraneuos labyrinth of second and fourth group rats (Figure 1, 2 and 3). Inflammation was not d etected in the parts of vestibulocochlear and facial nerves in meatus acousticus internus (Figure 4). The degeneration or lost of hairy cel ls of cochlea was not observed in any section prepared from group 2 and 4. Ornit hine did not reduce the harmful effect of sepsis on rats from group 4.
Immunohistochemical staining
p53 immunoreactive cells were not detected in any sections of the inner ear.
Plasma nitrate/nitrite and MDA levels
The mean plasma nitrite/nitrate and MDA levels were shown in table 1 (Table 1). When we compared the plasma levels of nitrite/nitrate and MDA
among groups, we found statistically significant differences: between groups 1
and 2 (MDA:p<0.001, nitrite: p>0.05, nitrate: p<0.005), groups 1 and 3 (MDA: p<0
.05, nitrite: p>0.05, nitrate: p<0.05), groups 1 and 4 (MDA: p<0.05, nitrite: p>
0.05, nitrate: p<0.05), groups 2 and 3 (MDA: p<0.001, nitrite: p<0.05, nitrate:
p<0.001), groups 3 and 4 (MDA: p<0.05, nitrite: p>0.05, nitrate: p<0.001). Howev
er, no significant difference was found in the comparison of groups 2 and 4 (MDA
: p>0.05, nitrite: p>0.05, nitrate: p>0.05).
 Büyütmek İçin Tıklayın |
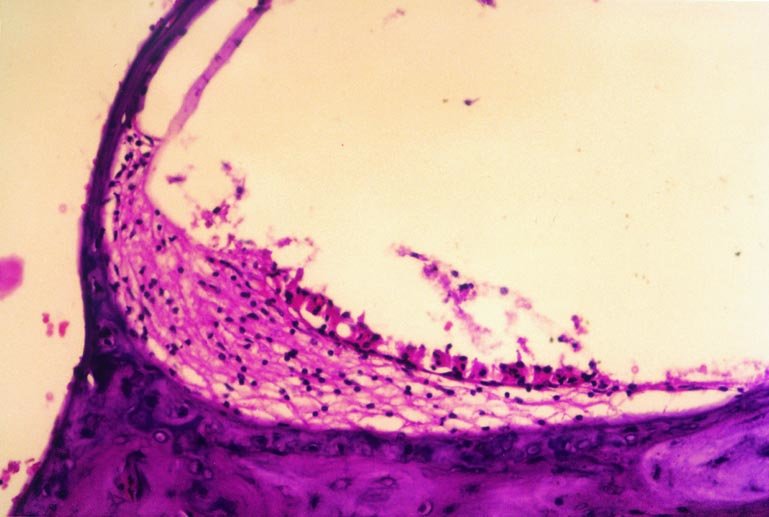
Figure 1: The normal appearance of cochleary section of control group |
 Büyütmek İçin Tıklayın |
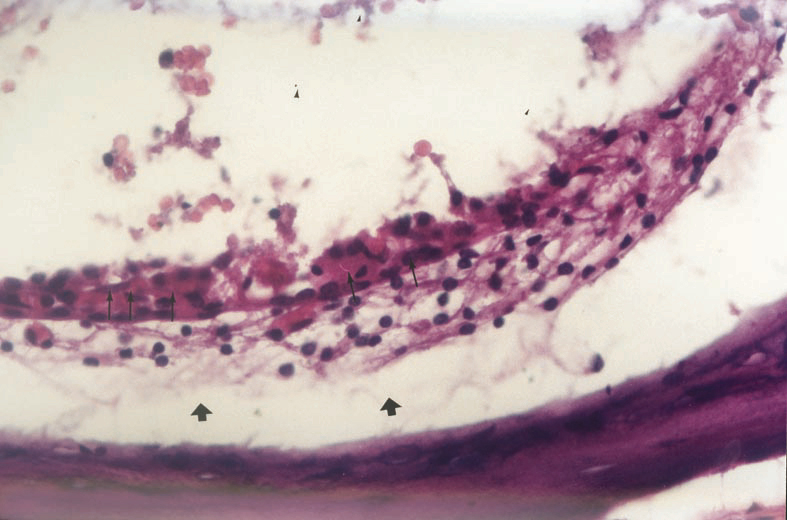
Figure 2: Congestion (narrow arrow) and subepithelial severe edema (wi de arrowhead) in stria vascularis of group 2 and 4. (H.E. x40) |
 Büyütmek İçin Tıklayın |
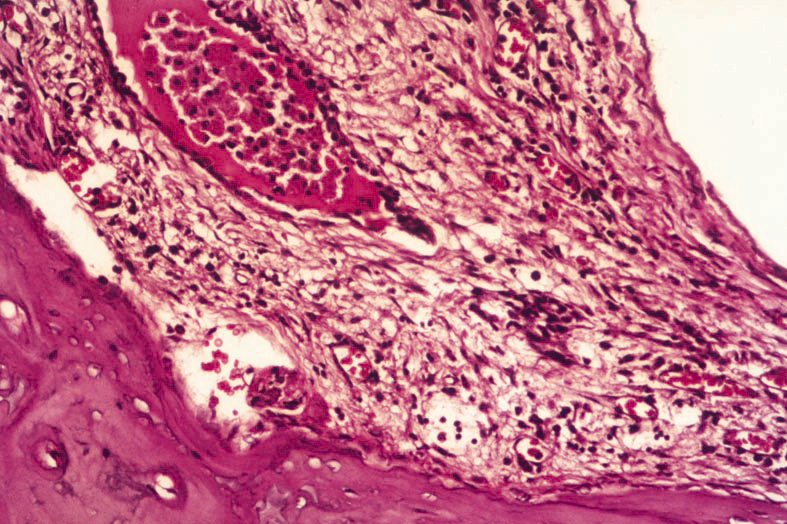
Figure 3: Periosteal intensive inflammation and neutrophil leukocyt in filtration in cochlea of groups 2 and 4. (H.E. x20) |
 Büyütmek İçin Tıklayın |
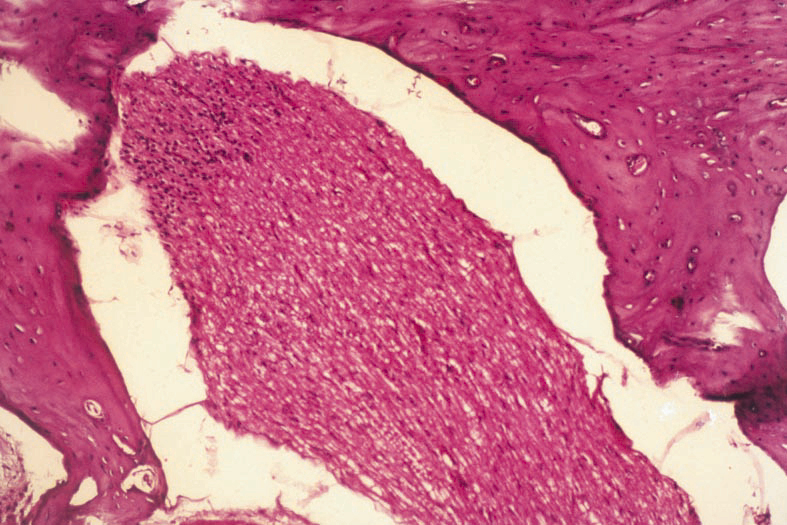
Figure 4: Normal appearance of meatus acousticuc internus and its cont aining in gruops 2 and 4 (H.E. x20) |
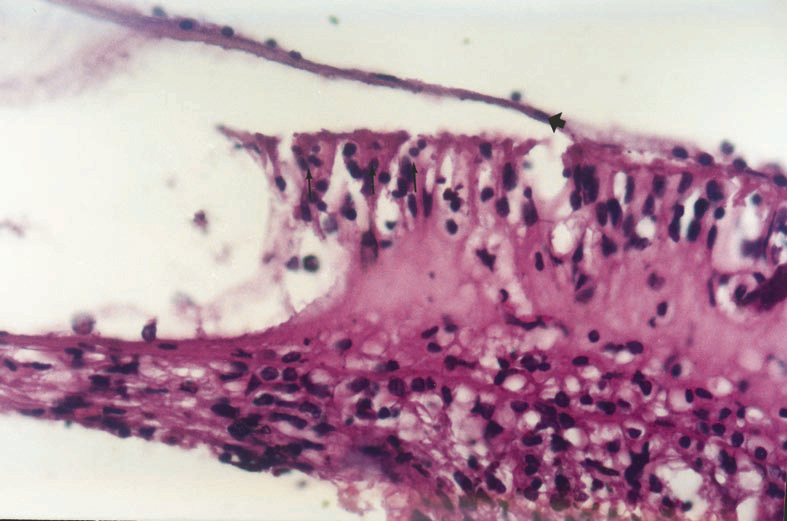 Büyütmek İçin Tıklayın |
Figure 5: View of normal appearance of tectorial membrane (wide arrow) , outer hair cells (narrow arrows) and basilar membrane |
Table 1: The mean±standart deviation of serum levels of nitrite/nitrate and MDA in groups
Discussion
In the present study, despite inflammation and edema commonly situated in modiolus, spiral ligament and stria vascularis, the degeneration or lost of hairy cell were not observed. The p53 immunoreactive cell was not detected in any section of the inner ear from animals of the second and the fourth group. This condition clearly supported the existence of blood-labyrinth barier in stria vascularis which protected the organ of corti against inflammation.The inner ear is enclosed with a bony capsule and lacks lymphatic drainage. The labyrinth is separated from the bloodstream by a barrier that helps to maintain the electrolytic features of the cochlear environment. This blood-labyrinth barrier presents tight junctions in the stria vascularis. It plays an important role in protection of cochlea during meningitis induced by LPS [11,13]. Some cytokines, such as TNF-a, interleukin-1 (IL-1), interferon-g (IFN-g), and various colony-stimulating factors are produced when there is a contact between LPS and host defense cells. During LPS induced endotoxemia, excessive NO formation by iNOS increases the expression of inflammatory markers. Massive intra- or extracellular NO generation by iNOS has been suggested to cause apoptosis [5]. Cytokine induced NO generation causes appereance of typical apoptotic features, DNA fragmentation, nuclear condensation and apoptotic body formation [6]. Tumor supressor gene product p53 has been shown to accumulate during NO mediated apoptosis in RAW 264.7 macrophages [5].
On the other hand, Suzuki et al [14] showed that the epithelia of the spiral modiolar vein (SMV) and collecting venule show an increase in expression of intercellular adhesion molecule-1 (ICAM-1) during inflammation. When the inflammatory stimuli causes DNA damage, the expression of p53 increases. There are two major functions of p53, repairing of DNA by stopping cell cycle, and leading cells to apoptosis. In our study, the p53 immunoreactive cells were not detected immunohistochemically. Hence we thought that the severity of cochlear inflammation either was not sufficient or bloodlabyrinth barier protected the inner ear from endotoxin to induce DNA damage in the inner ear cells of rats.
Because NO has been detected in the circulation and cerebrospinal fluid of septic patients, it has become the target of therapeutic strategies aimed at inhibiting iNOS or scavenging NO radicals with chemical moieties [1]. It was reported that L- ornithine prevented overproduction of NO by inhibiting transport of L-arginin [9]. Although L-ornithine is assumed to be a scavenging of NO, we could not observe this effect of L-ornithine biochemically and histopathologically. Furthermore, plasma nitrite/nitrate levels were reduced by the treatment of L-ornithine but this reduction was not significant statistically (p>0.05). This condition indicated either that the L-ornithine failed to prevent L-arginine transport to the cell or intracellular L-arginine storage is well preserved and thus not affected by the inhibition of L-ornithine in the endotoxemia.
CONCLUSION: Although inflammation is observed in different degrees especially in the stria vascularis and perimodiolar region, it does not spread to inner ear through the vestibulocochlear and facial nerve. The severity of cochlear inflammation was not sufficient to induce DNA damage in the inner ear cell. Additionally, it seems unlikely that L-ornithine at this dose can improve the manifestations of inflammation of inner ear during this type of endotoxemia.
Reference
1) Zanetti G, Baumgartner J D, Glaauser MP. Bacteremia, sepsis and septic shock. In Root RK, Waldvogel F, Corey L, Stamm WE, eds. Clinical Infectious Diseases. Oxford, Oxford University Press. 1999;471-481.
2) Morizono T, Ikeda K. Effect of Esherichia coli endotoxin on cochlear potentials following its application to the chinchilla middle ear. Eur Arch Otorhinolaryngol 1990; 247: 40-42. [ Özet ]
3) Petros A, Bennett D, Vallance P. Effect of nitric oxide sythase inhibitors on hypotension in patients with septic shock. Lancet 1991; 338: 1557-1558. [ Özet ]
4) Fang FC. Prospective series:host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 1997; 99: 2818-2825. [ Özet ]
5) Messmer UK, Ankorrona M, Nicotera P, Brune B. p53 expression in nitric oxide-induced apoptosis. FEBS Letters 1994; 355: 23-26. [ Özet ]
6) Brune B, Götz C, MeBmer UK, Sandon K, Hirvonen MR, Lapetina EG. Superoxide formation and macrophage resistanmce to nitric oxide mediated apoptosis. J Biol Chem 1997; 272(11): 7253-7258. [ Özet ]
7) Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, Billiar TR, Harris CC. Nitric oxide induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild type p53. Proc Natl Acad Sci USA Med Sci 1996; 93: 2442-2447. [ Özet ]
8) Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: Findings in normal and T- and B- cell deficient mice. Crit Care Med 1997; 25(8): 1298-1307. [ Özet ]
9) Bogle RG, Moncada S, Pearson JD, Mann GE. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br J Pharmacol 1992; 105: 768-770. [ Özet ]
10) Yagi K. Lipid peroxides and related radicals in clinical medicine. Edited by D.Armstrong. Free Radicals in Diagnostic Medicine Plenum Press, New York 1994:1-15.
11) Garcia Berrocal JR, Ramirez Camacho R. Immune response and immunopathology of the inner ear: an update. J Laryngol Otol 2000;114:101-107. [ Özet ]
12) Juhn SK, Rybak LP. Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol (Stockh) 1981;91:529-534. [ Özet ]
13) Suzuki M, Kitamura K, Nomura Y. Influence of changed blood PH on anionic sites in the labyrinth. Acta Otolaryngol (Stockh) 1995; 115:747-753. [ Özet ]
14) Suzuki M, Harris JP. Expression of intercellular adhesion molecule-1 during inner ear inflammation. Ann Otol Rhinol Laryngol 1995;104:69-75. [ Özet ]




