BIOMECHANICAL AND HISTOLOGICAL FEATURES OF COSTAL CARTILAGE GRAFT IN A RABBIT MODEL: COMPARATIVE RESULTS WITH THREE MONTHS OF FOLLOW-UP
2Department of Otolaryngology, Yeditepe University, Istanbul, Turkey
3Department of Metalurgical and Materials Engineering, Yildiz Technical University, Istanbul, Turkey
Summary
Objective: In this study, histological and biomechanical characteristics of rabbit rib cartilage used as a nasal dorsal graft were investigated.Material and Method: This prospective animal study included 8 male New Zealand rabbits. After harvesting rib cartilage, the graft was placed in the subperiosteal pocket made on the nasal dorsum. Contralateral 7th costal cartilages were also harvested as controls. On the third month after the procedure, the cartilage grafts and contralateral controls were removed and subjected to 3-point bending test. Hematoxylin-eosin stained sections were also used to evaluate chondrocyte viability and the status of the chondroid tissue.
Results: There was no statistical difference between the dimensions (thickness and width) of nasal dorsal graft and 7th costal cartilage. The flexural strength of the contralateral 7th costal cartilage and the number of viable chondrocyte cells were found to be significantly higher compared to nasal dorsal grafts (p=0.028, p=0.027, respectively)
Conclusion: Although we did not determine any significant reduction with time in the dimensions of costal cartilage grafts used in nasal surgery, it seems that biomechanical characteristics are altered as identified by the reduction in viable chondrocyte count and flexural strength.
Introduction
Cartilage grafts in nasal surgery, especially in rhinoplasty, are frequently required for both structural and aesthetic purposes. Autogenous cartilage grafts are preferred due to viral load of homografts and immune reactions frequent in alloplastic materials. An ideal cartilage graft material for nasal surgery must be biocompatible, easy to obtain, and should demonstrate strength while having sufficient elasticity [1,2].Although the main source of autogenous cartilage grafts is the nasal septum, in various conditions, including revision surgery, traumatic cases with reduced nasal tip projection, caudal septal weakness and/or saddle nose deformity, grafts are obtained from auricular or costal cartilage [3,4].
The nose, despite being located in an area open to external traumatic forces, has a unique elastic cartilage structure that can compensate against such forces. Therefore, correct cartilage graft selection is critical for the adequate reconstruction of this biomechanical structure in order to ensure long term success.
Previous cross-sectional studies showed that the 6th and 7th costal cartilages have similar biomechanical properties with the nasal septum [5,6]. Although several experimental histopathological studies have demonstrated long term cartilage viability with the use of autogenous costal cartilage [7-9], long term biomechanical results of such reconstructions have not been shown yet. It is also interesting that while some literature shows cellular loss of cartilage tissue that leads to recurrent deformation in the long term, other authors have stated that there was no significant cartilage resorption in long term follow up [7,10,11].
In this study, the histological and biomechanical characteristics of rabbit rib cartilage used as a nasal dorsal graft were investigated.
Methods
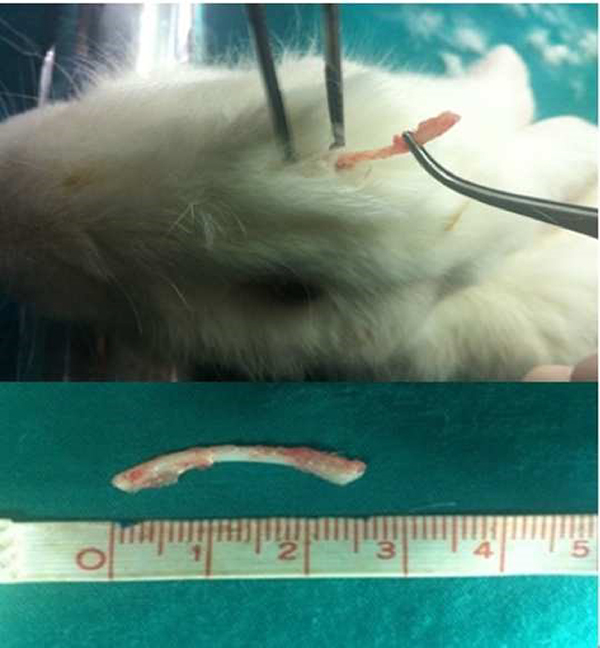
The study included 8 male (3200-3800 gram) New Zealand rabbits (Oryctolagos cuniculus). The Ethical Committee of xxxxxxxxxxxxxx approved the study (Date:10/11/2011, No:2011/155). Animals were received from the local laboratory of animal experiments and were housed in appropriately-sized pens in two groups (according to weight) during the 1-week habituation period during which they received scattered feeding with greens and hay, and standard pellet food. They were then transferred to single-animal cages inside the same room with water, scattered greens and standard pellet food. The light/dark cycle was 12/12 hours; temperature was 20-22 °C with 45% humidity.Animal Care and Procedures Under general anesthesia with 50 mg/kg ketamine hydrochloride and 10 mg/kg xylazine, the left thoracal region of each animal was shaved and sterilized. Rib cartilage harvesting method was performed as described previously be Coutt et al. [12]. A 2-cm horizontal incision was performed to the left inferior costal region and an approximately 2 cm section of the medial cartilaginous part of the 7th costal cartilage was resected with meticulous dissection, cleared from the surrounding soft tissue and perichondrium, and washed with isotonic sodium chloride (Figure 1). The width and thickness of all graft materials were measured by a digital caliper (Mitutoyo, Japan). The chest was closed with 4/0 polyglactin for muscular and dermis closure, and 4/0 silk for epidermal suture.
 Büyütmek İçin Tıklayın |
Figure 1: Harvesting of costal cartilage graft. 2cm horizontal 271 incision was performed to the left inferior costal region and approximately 2 cm long medial cartilaginous part of the 7th costal cartilage resected. |
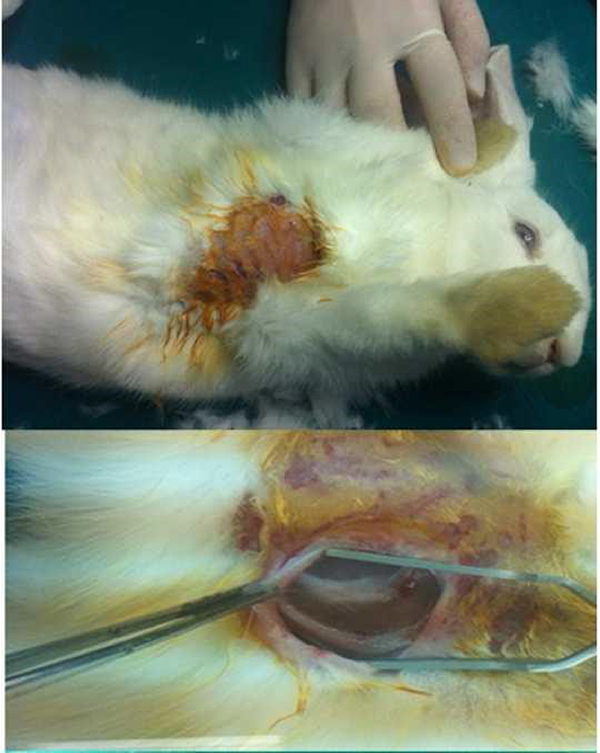
To prepare the recipient site, as previously described by Maas et al. [13], A 1-cm anterior incision through skin and subcutaneous tissues was made on the dorsum of the nose. A subcutaneous pocket extending 3 cm in the cranial direction was formed directly over the bony dorsum superficial to the periosteum. The cartilage graft was placed in this pocket and the incision was closed in one layer with 4/0 silk suture (Figure 2).
 Büyütmek İçin Tıklayın |
Figure 2: Placing of costal cartilage graft into the subcutaneous pocket over the nasal dorsum. |
Pain control that may occur in animals was provided by subcutaneous administration of 0.05 mg/kg buprenorphine hydrochloride before and after the operation every 12 hours for 3 days. Postoperatively, each rabbit was examined daily for signs of wound infection, seroma, hematoma, wound dehiscence, flap necrosis or pneumothorax until all wounds were healed. Subsequently, the animals were examined twice a week for 3 months. It has been previously shown that a 3-month follow up period in rabbits roughly corresponds to a 1-year follow-up in humans [14]. At the end of this period, the rabbits underwent anesthesia in a manner similar to the already described and then were sacrificed with intravenous administration of pentobarbital sodium (200 mg/kg). A midline incision was made along the nasal dorsum and cartilage grafts were removed, washed, dried and measured (width and thickness). Moreover, previous horizontal costal incision was extended through the contralateral side for harvesting contralateral 7th costal cartilages as controls. For histological examination, 0.5 cm-long specimens were harvested from both nasal dorsal graft and contralateral costal cartilages. The grafts were wrapped in saline impregnated gauze.
Mechanical Testing
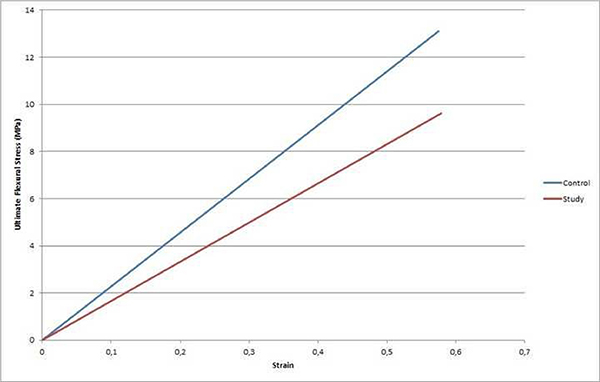
Three-point bending was carried out on a rectangular cross-section resting freely on two supports by means of the load acting on the specimen midway between the supports. The test specimen is deflected in this way at a constant rate until rupture occurs at the opposite (inferior) surface of the specimen (Figure 3). During this procedure, the force applied to the specimen and the resulting midspan deflection of the specimen was measured. The fracture formation was directly observed in all experiments and the force-deflection curve (mm) was automatically plotted. Flexural strength and elastic modulus were determined (Figure 4). Flexural strength can be defined as the ability of a material to withstand deformation under loading without failure. Elastic modulus, or Young's modulus, which was calculated from the force-deflection curve, describes tensile elasticity or the tendency of an object to deform along an axis when opposing forces are applied along that axis.
 Büyütmek İçin Tıklayın |
Figure 3: The test specimen is deflected in this way at a constant rate until rupture occurs at the other surface of the specimen. |
 Büyütmek İçin Tıklayın |
Figure 4: Fracture formation was observed in experiments. During this procedure force deflection curve (mm) was automatically plotted. |
All of the cartilage strips were subjected to the 3-point bending test. The strips were placed freely on supports that were 10 mm apart and the load applicator was located above the center of the strips. Deflection was imposed at a rate of 1 mm/s and the load was measured at a resolution of 0,001 N.
Histological examination
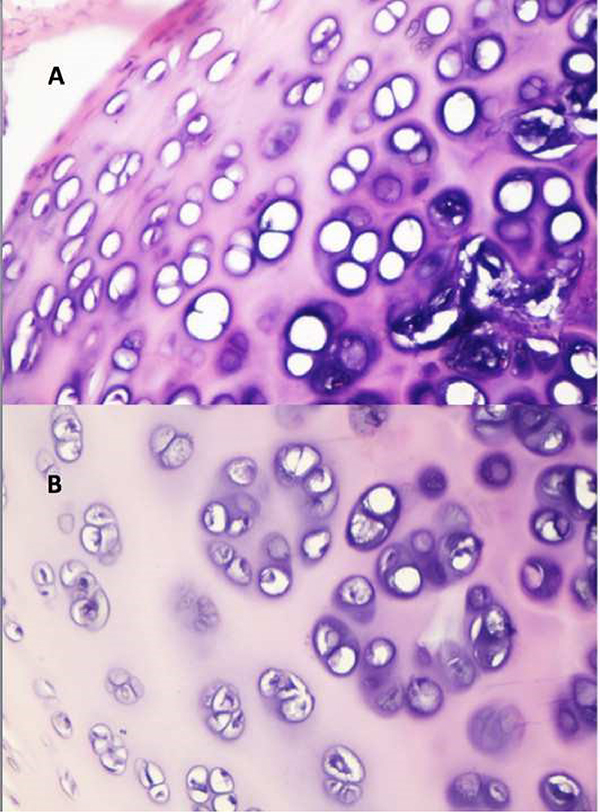
Hematoxylin-eosin stained sections were used to evaluate chondrocyte viability and the status of the chondroid tissue. The pathologist was blinded to the specimens. The specimens were fixed in 10% formaldehyde and then embedded in paraffin. Five micrometer thick horizontal 3 serial sections were performed from each specimen. The absence of chondrocyte nuclei was considered to indicate non-viable chondroid tissue. Viable cartilage cells were counted in 3 serial sections at 10 high-power field and the average of these results were calculated (Figure 5). Inflammation, osteogenesis, calcification, necrosis and fibrosis were evaluated and reported as present or absent.
 Büyütmek İçin Tıklayın |
Figure 5: A) Loss of viable chondrocytes with nuclei at high-power field and calcification in study group material B) Viable chondrocytes with nuclei at high-power field in control group material |
Statistical Analysis
All analyses were performed on SPSS v21 (SPSS Inc., Chicago, IL, USA). For the normality check, the Shapiro Wilk test and Q-Q plots were used. Data are given as mean ± standard deviation for continuous variables. Continuous variables were analyzed with the Mann Whitney U test. p<0.05 values accepted as statistically significant results.
Results
Eight animals were included in the study. Two subjects died on the 4th and 9th week of the follow-up period due to unknown reasons. Therefore, the 3 month follow up period was completed by 6 animals. The width, thickness, flexural strength, elastic modulus, and strain of nasal dorsal grafts and contralateral 7th costal cartilage are shown in Table 1. Mean thickness of nasal dorsal graft and contralateral 7th costal cartilage were 2.02 ± 0.39 mm and 2.05 ± 0.34 mm, respectively. Mean width of nasal dorsal graft and contralateral 7th costal cartilage were 2.4 ± 0.24 mm and 2.27 ± 0.3 mm, respectively. There was no statistical difference between the dimensions (thickness and width) of nasal dorsal graft and contralateral 7th costal cartilage (p=0.498, p=0.249, respectively) (Table 1). The flexural strength of the nasal dorsal graft and contralateral 7th costal cartilage were measured as 9.4 ± 3.6 MPa and 12.8 ± 3.47 MPa, respectively. We found that the flexural strength of the contralateral 7th costal cartilage was significantly higher compared to the nasal dorsal graft (p=0.028). Mean Elastic modulus obtained from the load deflection curve was 0.57 ± 0.1 N/m2 in nasal dorsal grafts and 0.58 ± 0.05 N/m2 in contralateral 7th costal cartilage (p=0.917) (Table 2).Table 1: Measurements of each nasal dorsal graft and each contralateral 7th costal cartilage
Table 2: Measurements of nasal dorsal grafts and contralateral 7th costal cartilages
There were no materials with histological calcification, osteogenesis and/or necrosis. However, nasal dorsal grafts of 3 subjects showed inflammatory reaction. In addition to this, the nasal dorsal grafts of 4 subjects had fibrosis around the grafted materials. Viable chondrocyte counting results revealed that nasal dorsal grafts had 25.1 ± 2.63 mean viable cells, while the contralateral 7th costal cartilages had 32.6 ± 3.01 mean viable cells. The number of viable chondrocyte cells in contralateral 7th costal cartilage were found to be significantly higher than that of nasal dorsal grafts (p=0.027) (Table 2).
Discussion
Exposure to various kinds of external forces, such as compression, tension, bending and/or combinations, are frequent in the nose. The nasal septum has a pivotal role in the resistance to these mechanisms [6]. Costal cartilage is mostly required in patients with inadequate nasal septum or severe septal deformations and/or patients who need more cartilage graft material for sufficient reconstruction. The primary aim is the creation of a stabilized nasal framework that is both aesthetically accurate and functional [15,16]. In this study, it was determined that there was no statistically significant difference in the dimensions of the costal cartilage used in nasal surgery over time. On the other hand, the elastic modulus and the number of viable chondrocyte cells decreased significantly over time when compared to the control group.Costal cartilage grafts can be used for both structural and aesthetic purposes in nasal dorsal otografts for the augmentation of the nasal dorsum and/or in L strut-shaped costal cartilages for structural support [15,16]. Long term results of costal cartilage grafts are often contradictory in the literature. Lattyak et al., in an animal model, reported a resolution rate of septal, auricular and costal cartilage with a frequency of 30.8%, 12.6% and 7.6%, respectively [7]. However, Wiseman et al. stated no resorption with viable chondrocytes in long term costal cartilage otografts [17]. Although, there are studies which show increased cartilage resorption rate in diced and crushed groups, some studies showed no difference in these groups [18,19]. Despite these conflicting results, histological examinations are utilized to assess viability and/or resorption rate in the majority of publications. In our study, we used both histological and mechanical bending parameters for the first time in the literature. Microcaliper results showed that there was no significant dimension change after 90 days in nasal dorsal cartilage grafts and contralateral controls. In addition, elastic modulus, a parameter that is usually substance-specific, was also determined to be unchanged in nasal dorsal grafts after 90 days. However, mechanical bending test revealed an average 26.56% reduction in the resistance of nasal dorsal cartilage graft deformation against applied load-compared to controls. Parallel to this, histological examination revealed an average 23% reduction in viable chondrocyte count in nasal dorsal cartilage grafts compared to controls.
The decrease in the modulus of elasticity may cause complications such as bending, displacement/extrusion and graft fracture and may have negative effects on clinical results. Many studies have reported these complications at various frequencies. In a systematic review of 21 different studies examining autologous costal cartilage procedures in rhinoplasty, Varadharajan et al. reported the following frequencies of complications: 5.2% warping, 0.6% displacement/extrusion, and 0.2% graft fracture at long-term [20]. In a meta-analysis examining the results of the use of autologous rib cartilage in rhinoplasty, Wee et al. found warping in 3.08% and displacement in 0.39%, while 14.07% of the patients required revision surgery in the long term. Consistent with our study, the authors reported that there were no dimensional differences in the graft after a follow-up of more than 1 year [21]. The long-term modulus of elasticity results of the graft could not be evaluated because previous studies were performed on humans. The fact that our study is an animal study is an important advantage that allows the graft to be removed and re-evaluated. In the current study, although no significant difference was found in the modulus of elasticity in the time interval examined, we thought that the results of the research, where longer term investigations will be conducted, may be a guide in determining the pathophysiology of the related complications.
It is remarkable that in the current study, the number of viable cartilage cells was statistically significantly lower in the nasal dorsal graft group compared to the control group. Although the lack of a similar study in the literature restricts any meaningful comparison, it was thought that a significant decrease in the count of viable cartilage cells may manifest itself clinically with a decrease in the strength of the graft, possibly leading to resorption or nasal fracture. As a matter of fact, these complications have been reported at various frequencies in various studies [20-23]. On the other hand, two surgical operations on the grettes in the nasal dorsal graft group may have affected the results. Because cartilage grafts, although they are immunologically privileged, have a high risk of resorption over time has been the biggest concern [24]. Experts try to solve this problem by choosing some modifications in graft preparation. In previous studies, it has been reported that mild to moderately crushed cartilage grafts give better results in terms of viability, flexibility, stability and proliferation rate compared to highly crushed grafts [25,26]. In addition, it has been reported that diced cartilage grafts with less stress applied to cartilage compared to crushed, fragmented cartilage grafts perform better with a higher number of living cells [27,28]. In this context, it was thought that higher exposure to trauma in grafts that underwent two surgical operations may increase apoptosis in the grafts and decrease the number of viable cells. Additionally, the results of homologous and autologous grafts were evaluated in other studies. In a meta-analysis comparing the results of rhinoplasty using autologous and homologous costal cartilage graft, Vila et al. reported that complications due to the modulus of elasticity of the graft and the count of viable chondrocytes were at similar frequencies in both groups [22]. Saadi et al. reported the same results in a similar study [23]. Although these studies suggest that autologous and homologous costal cartilages are not superior to each other, more studies are needed in this regard.
According to the results of this study, especially in structural reconstructive surgery, nasal septal grafts and/or columellar struts prepared from costal cartilages, alar cartilages and/or nasal dorsal onlay grafts may have a considerable risk for deformation due to the reduction of flexural strength by time. Therefore, increased bending moment of the costal cartilage grafts may improve long term results when relatively larger dimensions are used.
The most important limitation of our study is the small number of cases per group which was compounded by the fact that two animals were lost prior to final analyses. Secondly, although no subjects had graft necrosis in our study, 4 subjects had nasal dorsal grafts surrounded by fibrous tissue. We also did not determine the effect of fibrous tissue formation on chondrocyte viability and its role in providing additional mechanical support. Another limitation of the study is that the deflation rate limit (1 mm/sn) we used is higher (0.02 mm/sn) compared to other similar studies [29]. It is possible that this situation has reduced the sensitivity of the measurement. Finally, anatomical structures such as perichondrium, intercartilaginous ligaments, and surgical interventions such as transseptal and/or intercartilaginous sutures enabling a reduction on graft load were not assessed or applied in the current study.
In conclusion, although the dimensions of grafts remained similar at the end of follow-up, the chondrocyte loss identified via histological analysis may demonstrate underlying alterations in structural integrity. This possibility is supported by the decrease in flexural strength. New clinical studies are needed to determine the properties of the ideal costal cartilage graft required to minimize possible complications.
Conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Reference
1) Sherris DA. Relative benefits of the versatile autogenous costal cartilage graft in septorhinoplasty. Archives of Facial Plastic Surgery. 2002;4(3):177-179.
2) Sherris DA. Caudal and dorsal septal reconstruction: an algorithm for graft choices. American Journal of Rhinology. 1997;11(6):457-466.
3) Gunter JP, Rohrich RJ. Augmentation rhinoplasty: dorsal onlay grafting using shaped autogenous septal cartilage. Plastic and Reconstructive Surgery. 1990;86(1):39-45.
4) Velidedeoglu H, Demir Z, Sahin Ü, Kurtay A, Erol ÖO. Block and Surgicel-wrapped diced solvent-preserved costal cartilage homograft application for nasal augmentation. Plastic and Reconstructive Surgery. 2005;115(7):2081-2093.
5) Alkan Z, Acioglu E, Yigit O, Bekem A, Azizli E, Unal A, Sahin F. Determining the most suitable costal cartilage level for rhinoplasty: an experimental study. Otolaryngology--Head and Neck Surgery. 2012;146(3):377-381.
6) Alkan Z, Yigit O, Acioglu E, Bekem A, Azizli E, Kocak I, Unal A, Buyuk Y. Tensile characteristics of costal and septal cartilages used as graft materials. Archives of Facial Plastic Surgery. 2011;13(5):322-326.
7) Lattyak BV, Maas CS, Sykes JM. Dorsal onlay cartilage autografts: comparing resorption in a rabbit model. Archives of Facial Plastic Surgery. 2003;5(3):240-243.
8) Hizal E, Buyuklu F, Ozer O, Cakmak O. Effects of different levels of crushing on the viability of rabbit costal and nasal septal cartilages. Plastic and Reconstructive Surgery. 2011;128(5):1045-1051.
9) Sungur N, Koçer U, Uysal A, Arslan C, Kankaya Y, Sökmensüer C, Sökmensüer LK. Solvent dehydrated costal cartilage: evaluation in a rabbit model. Journal of Craniofacial Surgery. 2005;16(1):89-94.
10) Guyuron B, Friedman A. The role of preserved autogenous cartilage graft in septorhinoplasty. Annals of Plastic Surgery. 1994;32(3):255-260.
11) Sherris DA, Kern EB. The versatile autogenous rib graft in septorhinoplasty. American Journal of Rhinology. 1998;12(3):221-228.
12) Coutts RD, Woo S, Amiel D, Von Schroeder HP, Kwan MK. Rib perichondrial autografts in full-thickness articular cartilage defects in rabbits. Clinical Orthopaedics and Related Research. 1992(275):263-273.
13) Maas CS, Gnepp DR, Bumpous J. Expanded polytetrafluoroethylene (Gore-Tex soft-tissue patch) in facial augmentation. Archives of Otolaryngology?Head & Neck Surgery. 1993;119(9):1008-1014.
14) Rudderman RH, Guyuron B, Mendelsohn G. The fate of fresh and preserved, noncrushed and crushed autogenous cartilage in the rabbit model. Annals of Plastic Surgery. 1994;32(3):250-254.
15) Msc AM, Gantous A. The use of autogenous costal cartilage graft in septorhinoplasty. Otolaryngology?Head and Neck Surgery. 2007;137(6):862-867.
16) Araco A, Gravante G, Araco F, Castrì F, Delogu D, Filingeri V, Casciani C, Cervelli V. Autologous cartilage graft rhinoplasties. Aesthetic Plastic Surgery. 2006;30(2):169-174.
17) Wiseman JB, Holt GR, Keefe MA, Holck DE, Canaan RL, Clark WD. The fate of fresh, layered, nonsutured and sutured, autogenous cartilage in the rabbit model. Archives of Facial Plastic Surgery. 2000;2(4):256-259.
18) Cakmak O, Bircan S, Buyuklu F, Tuncer I, Dal T, Ozluoglu LN. Viability of crushed and diced cartilage grafts: a study in rabbits. Archives of Facial Plastic Surgery. 2005;7(1):21-26.
19) Buyuklu F, Hizal E, Yilmaz Z, Sahin FI, Cakmak O. Viability of crushed human auricular and costal cartilage chondrocytes in cell culture. Journal of Cranio-Maxillofacial Surgery. 2011;39(3):221-225.
20) Varadharajan K, Sethukumar P, Anwar M, Patel K. Complications associated with the use of autologous costal cartilage in rhinoplasty: a systematic review. Aesthetic Surgery Journal. 2015;35(6):644-652.
21) Wee JH, Park M-H, Oh S, Jin H-R. Complications associated with autologous rib cartilage use in rhinoplasty: a meta-analysis. JAMA facial plastic surgery. 2015;17(1):49-55.
22) Vila PM, Jeanpierre LM, Rizzi CJ, Yaeger LH, Chi JJ. Comparison of Autologous vs Homologous Costal Cartilage Grafts in Dorsal Augmentation Rhinoplasty: A Systematic Review and Meta-analysis. JAMA Otolaryngology?Head & Neck Surgery. 2020;146(4):347-354.
23) Saadi R, Loloi J, Schaefer E, Lighthall JG. Outcomes of Cadaveric Allograft versus Autologous Cartilage Graft in Functional Septorhinoplasty. Otolaryngology?Head and Neck Surgery. 2019;161(5):779-786.
24) Hapsari NP, Bangun K, Atmodiwirjo P, Ponco B, Dewi TIT, Halim J. The Effect of Perichondrium and Graft Modification on the Viability of Conchal Cartilage Graft: An Experimental Study in Rabbit. The Cleft Palate-Craniofacial Journal. 2021:1055665621998173. doi: 10.1177/1055665621998173.
25) Kayabasoglu G, Ozbek E, Yanar S, Sahin F, Keles ON, Yilmaz MS, Guven M. The comparison of the viability of crushed, morselized and diced cartilage grafts: a confocal microscopic study. European Archives of Oto-Rhino-Laryngology. 2015;272(5):1135-42.
26) Boccieri A, Marianetti TM, Pascali M. Crushed cartilage: a rescue procedure in rhinoplasty. Journal of Craniofacial Surgery. 2018;29(3):614-7.
27) Erol ÖO. The Turkish delight: a pliable graft for rhinoplasty. Plastic and reconstructive surgery. 2000;105(6):2229-41.




