THE EFFECT OF IRON DEFICIENCY ANEMIA ON OLFACTORY FUNCTION AND MUCOCILIARY CLEARANCE
2Ondokuz Mayıs Üniversitesi, İç Hastalıkları Anabilim Dalı, Samsun, Turkey
Summary
Objective: Iron is essential for various cellular functions, including enzymatic processes. This study aimed to evaluate olfactory and nasal mucociliary function in patients with iron deficiency anemia (IDA).Materials and Methods: The study included 35 IDA patients (IDA Group) and 24 healthy subjects (Control Group). In order to exclude hormonal effects related to gender and menopause, all individuals in the groups were women between the ages of 18-45. Complete blood count tests of the patients were tested. Subjects were asked to evaluate smell functions a visual analog scale (VAS). The olfactory function was measured with Sniffin' Sticks odor test consisting of three subtests: odor threshold (T), odor discrimination (D), and odor identification (I). Sodium saccharin test was used to measure the mucociliary clearance (MCC) time.
Results: Hemoglobin, hematocrit, mean cell volüme (MCV), and mean cell hemoglobin (MCH) values were significantly lower in IDA group than in the control group (p<0.001). There was no difference between the groups in terms of MCC time (p=0.174). IDA group had lower VAS-Smell scores (p=0.012). The mean T, D, I, and TDI scores of the Sniffin" Sticks test were significantly lower in the IDA group (p<0.001, p=0.002, p<0.001, p<0.001, respectively). The linear regression analysis of the independent variables affecting the TDI value was not statistically significant (F=1.334, p=0.271).
Conclusion: In this study, IDA group had significantly worse olfactory function. We thought that olfactory dysfunction in patients with IDA was related to iron deficiency and related enzymatic factors.
Introduction
The World Health Organization (WHO) has recognized iron deficiency anemia (IDA) as the most common nutritional deficiency in the world[1]. Iron is vital for many physiological processes, and its deficiency causes various health consequences[2]. IDA symptoms include fatigue, cold intolerance, poor mental performance, dyspnea, and dysphagia due to anemia, as well as decreased mental-motor functions, neurological, cardiological, and gastrointestinal system problems, and auditory and visual function losses due to iron deficiency[3].The sense of smell influences hygiene and eating habits and provides as a warning system for potential life-threatening hazards such as spoiled food, gas leaks, and fires[4]. Anatomical and neurological pathologies that prevent odor molecules from reaching the olfactory area or central transmission lead to olfactory dysfunction. The effects of IDA on olfactory function have previously been evaluated in an experimental rat study, and behavioral olfactory dysfunction has been found in iron-deficient rats[5]. There is only one study that has clinically evaluated the effect of IDA on olfactory function, and it was shown that olfactory functions were impaired in this study[6].
Respiratory ciliary activity is defined as the removal of foreign particles from respiratory mucosal surfaces and the ability of mucosal surfaces to retain moisture. Ciliary functions can be assessed by nasal mucociliary clearance (MCC), which is measured by determining the time taken to eliminate inhaled aerosols[7]. Nasal MCC is affected by many factors, such as age, hormonal status, sinonasal diseases, nasal trauma, sinonasal surgeries, upper respiratory tract infection, toxins, drugs, ambient temperature, and active-passive smoking[8-11]. As the ciliary activity develops as a result of a series of enzymatic reactions, it has been suggested that micronutrient deficiencies can impair MCC. Although it has been shown that deficiency in various nutrients can affect nasal mucociliary functions, no study evaluating the nasal mucosal function of iron deficiency has been found in the literature.
This study aimed to evaluate the olfactory and nasal mucociliary function in patients with IDA.
Methods
This prospective case-control study was conducted at Ondokuz Mayıs University Department of Otorhinolaryngology between January 2021- January 2022. The study was performed under the Helsinki Declaration after obtaining approval from the local ethics committee (OMU-KAEK2020/612). All participants were informed with a written consent form.Participants: The study included 35 IDA patients (IDA Group) and 24 healthy subjects (Control Group). In order to exclude hormonal effects related to gender and menopause, all individuals in both the IDA and control groups were women between the ages of 18-45. The diagnosis of IDA was made with hemoglobin, hematocrit, mean cell volume (MCV) and mean cell hemoglobin (MCH), serum iron, total iron binding capacity (TIBC), and ferritin values, taking into account World Health Organization (WHO) criteria[12]. Healthy adult women with normal hemogram findings (hemoglobin, hematocrit, MCV and MCH) formed the control group. Patients who had upper respiratory tract infection in the last month, sinonasal diseases, nasal surgery history, nasal-head trauma history, malignancy history, systemic or neurodegenerative disease history and smoker, pregnant, breastfeeding and menopausal patients were excluded from the study. Nasal and nasopharyngeal findings of all participants were normal.
Clinical Data: Complete blood count tests, hemoglobin, hematocrit, MCV and MCH values of the patients in both groups were tested. Additionally, serum iron, TIBC, ferritin and iron saturation values of the patients in the IDA group were also measured.
Subjective assessment of olfactory function: Patients in IDA and Control Group were requested to measure olfactory functions using a visual analog scale (VAS) (VAS-Smell; 0: total loss of smell, 10: excellent sense of smell).
Psychophysical assessment of olfactory function: The olfactory function was measured using Sniffin' Sticks odor test (Sniffin' Sticks, Burghart Messtechnik GmbH, Wedel, Germany), a validated olfactory test consisting of three subtests: threshold (T), discrimination (D), and identification (I).
Odor threshold test: 16 triplets of fragrance pens with one n-butanol pen and two solvent pens were presented in increasing odor concentration. After correctly identifying the odor-containing pen twice in a presented triplet, the test continued until the patients could no longer correctly identify the odor-containing pen. The test was repeated seven times without reversals detected. The threshold score was calculated as collected from the last four thresholds and divided into four.
Odor discrimination test: Triple odor pens, two with the same and one with a different odor, were used and the patients were asked to find the different odor. The discrimination score was the sum of correctly identified pens.
Odor identification test: Sixteen pens containing odors known to the general public were used, and patients were asked to identify the odor from four options. Sum of correct answers represented the identification test score.
The sum of the three subset scores is called the TDI score. TDI score ?15 was anosmia, between 16 and 30 was hyposmia, and ≥31 was normosmia[13].
Mucociliary clearance time: Sodium saccharin test was used to measure the MCC time. Participants were instructed to not eat or drink anything 30 minutes before the test. Patients were asked to sit upright. A 1 mm diameter sodium saccharin tablet was placed into the nasal cavity, 1 cm posterior to the anterior border of the inferior turbinate. Participants were asked to continue sitting upright and asked not to breathe deeply, sniff, speak, or cough. The time from the insertion of the saccharin tablet to the perception of the sweet taste in the oropharynx was recorded as MCC time. The mean normal MCC time is 9-17 min, < 9 min is short, and >17 min is long[8].
Statistical Analysis:
Statistical analyses were performed with SPSS version 23.0 (IBM Corp., Armonk, NY, USA). Shapiro-Wilk, Kolmogorov-Smirnov tests and skewness-kurtosis statistics were used to assess normal distribution. Categorical variables were analyzed using Pearson Chi-Square test. Independent sample t-test was used for normally distributed data. Linear regression analysis was used to examine the variables affecting the TDI value. The significance level was taken as p<0.05.
Results
The age, hemoglobin, hematocrit, MCV, and MCH values of the groups are given in Table 1. The IDA Group included 35 female patients (mean age=34.54 ± 9.3). The Control group included 24 female patients (mean age=33.79 ± 9.32). There was no statistically significant difference between the two groups in terms of age (p= 0.762). Mean hemoglobin values were 10.11 ± 1.67 g/dl and 13.16 ± 0.65 g/dl, hematocrit values were 32.33 ± 2.58% and 38.29 ± 1.73%, MCV was 73.73 ± 7.61 fL and 85.04 ± 3.5 fL and MCH was 23.17 ± 3.69 pg and 29.25 ± 1.45 pg in IDA and control group respectively. Hemoglobin, hematocrit, MCV, and MCH values were significantly lower in IDA group than in the controlgroup (p<0.001).Table 1: Demographic and Hematologic Features of Groups
MCC time was 12.4 min in the IDA group and 11.17 min in the control group aand there was no statistically difference between groups (p=0.174). IDA group had lower VAS-Smell scores (8.66 ± 1.11) than in control group (9.38 ± 0.92) (p=0.012). The mean T, D, I and TDI scores of the Sniffin" Sticks test were were significantly lower in IDA group than control group (threshold 4.24 ± 1.94 vs. 6.56 ± 1.8, p<0.001; discrimination 11.43 ± 2.03 vs. 12.88 ± 1.33, p=0.002; identification 11.23 ± 2.09 vs. 13.29 ± 1.71, p<0.001; TDI score 26.9 ± 4.33 vs. 32.73 ± 3.42, p<0.001) (Table 2).
Table 2: Assessment of Mucociliary Clearance Time and Olfactory Functions of Groups
MCC time was normal range in 28 (80%), short in 4 (11.4%), long in 3 patients (8.6%) in the IDA group and in the control group, 19 (79.2%) were normal and 5 (20.8%) were short, and there was no statistically difference between the two groups (p=0.236). According to TDI scores, there were no anosmic patients in either group. Thirty of the patients (85.7%) in the IDA group were hyposmic and 5 (14.3%) were normosmic. Eight of the patients (33.3%) in the control group were hyposmic and 16 (66.7) were normosmic. The olfactory status of patients were significanty different in the two groups (p<0.001) (Figure 1).
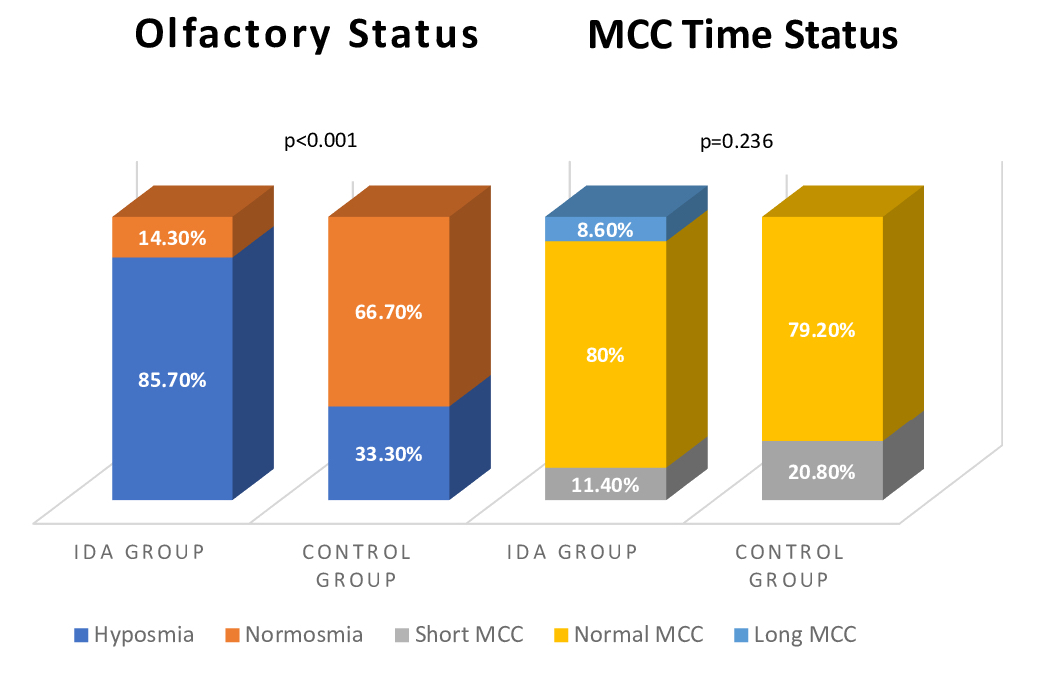 Büyütmek İçin Tıklayın |
Figure 1: Olfactory and Mucociliary Clearance Time Status of Groups. |
Serum iron, TIBC, ferritin and iron saturation, vitamine B12 and folic acide values of the patients in the IDA group were also measured. Mean ferritin was 7.44 ± 4.96 ng/mL, mean iron was 29.33 ± 11.2 µg/dL, mean iron saturation was 7.15 ± 3.13 %, mean TIBC was 443.46 ± 48 µg/dL, mean vitamine B12 was 307.85 ± 88.81 pg/mL and mean folic acide was 6.65 ± 2.71 ng/mL. All values were lower than normal range.
The linear regression analysis of the independent variables (hemoglobin, hematocrit, MCV, MCH, serum iron, iron satutarion, TIBC, ferritin, B12, FA) affecting the TDI value was examined, and the linear regression model established was not statistically significant (F=1.334, p=0.271) (Table 3). Hemoglobin, mean cell hemoglobin, and iron saturation independent variables were omitted because they cause multicollinearity problems in the model.
Discussion
In our study, female patients with iron deficiency anemia and healthy women without any systemic disorders were compared in terms of olfactory function. The IDA group consisted of patients diagnosed with iron deficiency anemia according to the WHO criteria, and there was a significant difference between the two groups in terms of all hematologic parameters. The olfactory functions of the participants in the groups were evaluated subjectively using the VAS and psychophysically using the Sniffin' Sticks olfactory test. VAS smell scores; the Sniffin' Stick test"s T (Threshold), D (Discrimination), and I (Identification) values; and the TDI value obtained by totaling the three subtest scores were significantly lower in the IDA group than in the control group. Patient evaluations without an objective test may sometimes not coincide with the actual results. In our study, although VAS-Smell scores were significantly lower in the IDA group, VAS scores were close to each other. Sniffin Sticks' test results, which psychophysically evaluate olfactory function, were significantly lower in the IDA Group. These results eliminate the concerns about 'clinical value' arising from the closeness of the patient evaluation results.When iron intake is insufficient to meet requirements and compensate for physiological or pathological losses, body iron stores are depleted. The most common causes of iron deficiency are increased requirements, decreased dietary intake, malabsorption, chronic blood loss such as gastrointestinal bleeding and menstruation, and drug-related losses[3]. Iron is essential for a variety of cellular functions, including enzymatic processes in almost all metabolic pathways, oxygen transport, DNA synthesis, and mitochondrial energy production[14,15]. Therefore, patients with IDA develop iron deficiency-specific symptoms along with anemia-related symptoms. Iron deficiency especially affects epithelial cells, causing dry skin, dry hair, hair loss, and koilonychia. In addition, there may be loss of tongue papillae and atrophic glossitis in severe cases[14]. Some studies have reported a significantly reduced quality of life of patients with IDA, even if they are asymptomatic, and that this reduction is related to both symptom-dependent and general well-being factors. In a study, it was observed that the vitality and general health status of Japanese women with IDA were below normal limits and both hemoglobin levels and physical function, vitality and general health perception improved with treatment[16]. One of the many factors affecting the quality of life is the sense of smell. In particular, the impact of the sense of smell on quality of life is greater in women[17]. In this study, we found that there was a significant loss of smell in patients with anemia. Although patients are often unable to explain the reasons for the deterioration in quality of life, we believe that loss of smell also impairs general well-being.
Olfactory mucosa and sensory neurons are important for olfactory function. The nasal mucosa provides a favorable environment for olfactory signal transduction via mucociliary clearance by producing odor-binding proteins[18]. Considering the effect of iron deficiency on epithelial cells and papillae of the tongue, the MCCs of the patients were also evaluated to assess whether a possible cause of olfactory dysfunction was the nasal mucosa. But the fact that the MCC in the patient and control groups was similar ruled out nasal mucosal effects.
As in our study, iron deficiency leading to olfactory dysfunction may be explained by different theories. Considering that iron plays a role in many neuronal enzymatic events, enzymatic slowing of olfactory transmission in the case of its deficiency may result in olfactory dysfunction[5]. Olfactory stimulation requires a series of enzymatic and chemical reactions. After odorants induce a stimulus in the olfactory receptor neurons, nitric oxide synthase (NOS) is activated first. Heme (iron-bound porphyrin), a component of hemoglobin, is a cofactor for NOS activity, mediating the exchange of GABA and glutamate via NMDA receptors[5]. Iron deficiency reduces NOS activity in this first step of olfactory stimulation. Iron is also a cofactor for tyrosine hydroxylase (TH), a key enzyme in the neuronal synthesis of dopamine, which controls glutamate release from olfactory receptor neurons. In addition, tryptophan dioxygenase (TDO), indoleamine 2,3-dioxygenase (IDO), and 3-hydroxyalkylanic acid oxygenase (3-HAO), which are enzymes involved in the synthesis of quinolinic acid that activates NMDA receptors in mitral and granule cells are iron dependent[5]. As a result, NOS, TDO, IDO, 3-HAO, and TH enzyme activities decrease in cases of iron deficiency. Accordingly, it is thought that problems occur at different steps of the signaling pathway, and olfactory dysfunction develops[5]. In their experimental study, Kumara et al. observed that the olfactory behavior of rats with iron deficiency was significantly altered, and they attributed this dysfunction to issues in the enzymatic pathway mentioned above[5]. In the only clinical study in the literature on this subject, Dinç et al. [6], evaluated olfactory function using the Sniffin' Sticks test battery, as in our study, and reported that although T and TDI values were significantly lower in women with IDA than in the control group, there was no difference between the groups in terms of the I and D subtests. In our study, all subtests and TDI values were significantly lower in IDA group compared to the control group.
In our study, unlike the study by Dinç et al. [6], the most effective hematologic parameters on TDI were evaluated by linear regression analysis, and none of these parameters were found to be effective on TDI. However, hemoglobin, MCH, and serum iron independent variables were excluded because they caused a multicollinearity problem in the model. Although the effect of iron on olfactory function could not be evaluated using regression analysis, the fact that other hematologic parameters were not found to be effective on TDI suggested that olfactory dysfunction was related to iron deficiency and associated enzymatic factors, like the study of Kumara et al. [5].
In addition to its many metabolic effects, iron deficiency is also known to negatively affect cognitive functions. In a study, it was reported a positive association between anemia, cognitive decline, and dementia in people over the age of 65 years[19]. In another study, it was reported that iron deficiency in the perinatal period may be associated with delayed neurocognitive development and psychiatric diseases[20]. Even if it did not lead to anemia, it was found that low iron stores were significantly associated with poorer performance in the cognitive executive planning function in educated young women[21]. Numerous studies on smell and cognitive function report a strong relationship between each other. In a meta-analysis, it was reported that there was an association between mild cognitive impairment and olfactory dysfunction, and that especially the Olfactor Identification tests are impaired. It has been suggested that identification tests can be used as a screening test for people at risk of Alzheimer's disease[22]. Although we did not analyze the cognitive status of the patients in our study, the fact that all the patients were young and had no known systemic, neurological, or psychiatric diseases reduces the risk of cognitive impairment.
In addition, in order to exclude factors affecting olfactory and cognitive functions such as age, hormonal status and gender, the evaluation of female patients between the ages of 18-45 who had not reached menopause and were of reproductive age ensured homogeneity within and between groups. Even if they were non-menopausal, patients over 45 were excluded from the study to avoid being affected by age-related change bias, and homogeneity within and between groups was supported.
However, this age and gender restriction made to ensure homogeneity caused the number of participants to be limited, which is one of the limitations of this study. Another limitation of our study is that the neurocognitive states of the patients were not evaluated with questionnaires or imaging methods. We believe that our study will be a reference for such studies to be conducted with larger patient groups in the future.
Conclusion
In conclusion, we found that female patients with IDA had significantly worse olfactory function than healthy control patients of similar age group, and most of the patients had hyposmia. When the hematologic parameters that most affected olfactory function were evaluated, it was observed that no hematologic value affected olfactory function.
DECLARATIONS
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval: Institutional ethical approval was obtained with the number of OMUKAEK 2020/612. All procedures performed in this study involving human participants were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. The study was conducted at Ondokuz Mayıs University School of Medicine Department of Otolaryngology.
Consent to participate: Written informed consent was obtained.
Consent for Publication: The authors transfer all copyright ownership of the manuscript to the "Turkish Archives of Otorhinolaryngology" in the event the work is published.
Conflict of Interest Statement: The authors declare there are no conflicts of interest - financial or otherwise - related to the material presented herein.
Previous Presentation: Not applicable
Peer-review: Externally peer-reviewed
The authors warrant that the article is original, is not for consideration by another journal, has not been previously published in a journal, and has been prepared according to the manuscript rules as described in your journals" instructions page. This submission was read and approved by all of the authors. The authors are responsible take for the integrity of the content of the manuscript.
Reference
1) Kumar A, Sharma E, Marley A, Samaan MA, Brookes MJ. Iron deficiency anaemia: pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022;9(1): e000759. [ Özet ]
2) Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. 2021;397(10270):233-48. doi: 10.1016/S0140-6736(20)32594-0. [ Özet ]
3) Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67. [ Özet ]
4) Altundag A, Ay SA, Hira S, Salihoglu M, Baskoy K, Deniz F, Tekeli H, Kurt O, Yonem A, Hummel T. Olfactory and gustatory functions in patients with non-complicated type 1 diabetes mellitus. Eur Arch Otorhinolaryngol. 2017;274(6):2621-7. [ Özet ]
5) Kumara VR, Wessling-Resnick M. Influence of iron deficiency on olfactory behavior in weanling rats. Journal of behavioral and brain science. 2012;2(2):19552. [ Özet ]
6) Dinc ME, Dalgic A, Ulusoy S, Dizdar D, Develioglu O, Topak M. Does iron deficiency anemia affect olfactory function? Acta Oto-Laryngol. 2016;136(7):754-7. [ Özet ]
7) Akcan FA, Dündar Y, Akcan HB, Uluat A, Cebeci D, Ünlü İ. Evaluation of nasal mucociliary clearance time in patients with Vitamin-D deficiency. European Archives of Oto-Rhino-Laryngology. 2019;276:1075-80. [ Özet ]
8) Habesoglu M, Demir K, Yumusakhuylu AC, Yilmaz AS, Oysu C. Does Passive Smoking Have an Effect on Nasal Mucociliary Clearance? Otolaryng Head Neck. 2012;147(1):152-6. [ Özet ]
9) Munkholm M, Mortensen J. Mucociliary clearance: pathophysiological aspects. Clinical physiology and functional imaging. 2014;34(3):171-7. [ Özet ]
10) Horasanli E, Acar A, Muslu B, Çayönü M, Cimencan M, Kayabaşi S. Assessment of nasal mucociliary clearance in anesthetists. Turkish Journal of Medical Sciences. 2015;45(1):197-201. [ Özet ]
11) Soylu Özler G, Akbay E, Akkoca AN, Karapınar OS, Şimşek GÖ. Does menopause effect nasal mucociliary clearance time? European Archives of Oto-Rhino-Laryngology. 2015;272:363-6. [ Özet ]
12) Force UPST. Screening for iron deficiency anemia?including iron prophylaxis. Guide to Clinical Preventive Services: Report of the US Preventive Services Task Force 2nd edition: Williams & Wilkins; 1996.
13) Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, Seeber H, Pauli E, Hummel T. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. European Archives of Oto-Rhino-Laryngology. 2000;257(4):205-11. [ Özet ]
14) Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387(10021):907-16. [ Özet ]
15) Cappellini MD, Comin?Colet J, de Francisco A, Dignass A, Doehner W, Lam CS, Macdougall IC, Rogler G, Camaschella C, Kadir R, Kassebaum NJ, Spahn DR, Taher AT, Musallam KM. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. American journal of hematology. 2017;92(10):1068-78. [ Özet ]
16) Ando K, Morita S, Higashi T, Fukuhara S, Watanabe S, Park J, Kikuchi M, Kawano K, Wasada I, Hotta T. Health-related quality of life among Japanese women with iron-deficiency anemia. Quality of life research. 2006;15:1559-63. [ Özet ]
17) Zou L-Q, Hummel T, Otte MS, Bitter T, Besser G, Mueller CA, Welge-Lu?ssen A, Bulut OC, Go?ktas Ö, Negoias S, Li S-B, Haehner A. Association between olfactory function and quality of life in patients with olfactory disorders: a multicenter study in over 760 participants. Rhinology. 2021;59(2):164-72. [ Özet ]
18) Patel RM, Pinto JM. Olfaction: anatomy, physiology, and disease. Clinical anatomy. 2014;27(1):54-60. [ Özet ]
19) Andro M, Le Squere P, Estivin S, Gentric A. Anaemia and cognitive performances in the elderly: a systematic review. Eur J Neurol. 2013;20(9):1234-40. [ Özet ]
20) Radlowski EC, Johnson RW. Perinatal iron deficiency and neurocognitive development. Frontiers in human neuroscience. 2013;7:585. [ Özet ]
21) Blanton CA, Green MW, Kretsch MJ. Body iron is associated with cognitive executive planning function in college women. British journal of nutrition. 2013;109(5):906-13. [ Özet ]
22) Roalf DR, Moberg MJ, Turetsky BI, Brennan L, Kabadi S, Wolk DA, Moberg PJ. A quantitative meta-analysis of olfactory dysfunction in mild cognitive impairment. Journal of Neurology, Neurosurgery & Psychiatry. 2017;88(3):226-32. [ Özet ]




