AURICULAR CUTANEOUS LEISHMANIASIS: A RETROSPECTIVE EVALUATION OF 12 PATIENTS
Summary
Aim: Leishmaniasis refers to a group of diseases caused by protozoan parasites belonging to the genus Leishmania. Cutaneous leishmaniasis (CL) lesions are typically observed as single or multiple lesions, particularly in the head-neck region. Auricular involvement in CL is extremely rare. The aim of this study is to examine the clinical features, diagnosis, and treatment methods of 12 patients diagnosed with auricular CL.Material and methods: In this retrospective study, the files of 12 patients diagnosed with CL through clinical and microscopic examination, and presenting with auricular involvement, were reviewed at our Dermatology and Venereology clinic between October 2019 and October 2022. Clinical and demographic features such as age, gender, number, localization, size, and duration of lesion, and treatments received were recorded from the patients' files.
Results: All patients diagnosed with CL were male (100%). The mean age of patients was 15.41±14.94. All patients presented with involvement of a single ear. Helix involvement was observed in 6 (50%) patients, antihelix in 4 (33%) patients, and lobule in 2 (17%) patients. The average duration of lesions was 4.6±2.8 months. The average size of the lesions was 3.4±2.3 cm. Cutaneous leishmaniasis diagnosis was made by direct microscopic examination in all patients, and all were treated with meglumine antimoniate (MA) at a dose of 20 mg/kg/day for 20 days intramuscularly. No cutaneous or systemic side effects related to MA treatment were observed in any patients.
Conclusion: The possibility of auricular involvement in CL should be kept in mind, and the diagnosis should be confirmed by microscopic examination. Systemic MA treatment should be considered in patients with auricular region involvement.
Introduction
Cutaneous leishmaniasis (CL), caused by Leishmania protozoa, is a disease transmitted through the bites of infected Phlebotomus genus sandflies[1]. Although CL has a wide variety of clinical presentations, the most common form is ulcerative crusted nodules and plaques that heal spontaneously leaving scars[2-4]. Treatment in CL varies according to the location of the lesion, its size, and the patient's immune status. Treatment goals are to minimize local damage and functional losses, accelerate healing, and reduce the possibility of recurrence. The most preferred drugs in the treatment of CL are pentavalent antimony compounds such as meglumine antimonate and sodium stibogluconate[1,2,5] Lesions in CL are most frequently seen in the head-neck region as single or multiple lesions[5-7]. A type of ear leishmaniasis caused by Leishmania mexicana, commonly found in Mexico and Central America, is known as "Chiclero's ulcer," primarily affecting the ears of forest workers. However, ear leishmaniasis in the Old World is quite rare[8,9]. Cutaneous leishmaniasis in this location can be mistaken for malignancies, other infectious diseases, and inflammatory processes. Therefore, particularly in endemic areas for CL, cutaneous leishmaniasis should be considered in the differential diagnosis of ulcerative lesions and auricular enlargements[8-11]. Despite its rarity, delayed diagnosis and treatment of auricular involvement can lead to destruction in the ear[9,12-14].The aim of this study is to examine the clinical characteristics, diagnosis, and treatment methods of 12 patients diagnosed with auricular CL between October 2019 and October 2022.
Methods
In this retrospective study, the files of patients diagnosed with CL through clinical and microscopic examination, were reviewed at our Dermatology and Venereology clinic between October 2019 and October 2022. The files of 1784 patients diagnosed with CL were examined retrospectively, and 12 patients with auricular involvement and no missing data in their files were included in the study.Clinical and demographic features such as age, gender, number, localization, size, and duration of lesion, and treatments received were recorded from the patients' files. Lesion size was determined by measuring the diameter of the lesion. Diagnosis of CL was made by observing amastigotes in dermal scrapings from solid lesions like papules, nodules, plaques, and in smear materials from crusted ulcerative lesions. Systemic meglumine antimonate (MA) therapy was administered at a dose of 20 mg/kg/day for 20 days intramuscularly (IM). In patients treated with systemic MA, hemogram, lipase, amylase, and renal and liver function tests were checked weekly for the first week and then bi-weekly. Additionally, electrocardiograms were conducted every other day in the first week and then twice a week.
Statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation (SD), and categorical data as frequency (%).
Ethical approval for the present study was obtained from the local ethics committee (Number: 23.20.23/2023).
Results
All patients diagnosed with CL were male (100%). The mean age of patients was 15.41±14.94. All patients presented with involvement of a single ear. Helix involvement was observed in 6 (50%) patients, antihelix in 4 (33%) patients, and lobule in 2 (17%) patients (Figure 1). The average duration of lesions was 4.6±2.8 months. The average size of the lesions was 3.4±2.3 cm. CL diagnosis was confirmed by direct microscopic examination in all patients.(Figure 2) All patients were treated with IM MA at a dose of 20 mg/kg/day for 20 days. No cutaneous or systemic side effects related to MA treatment were observed in any patients. Complete healing of lesions was observed in all patients after 12 weeks of treatment.
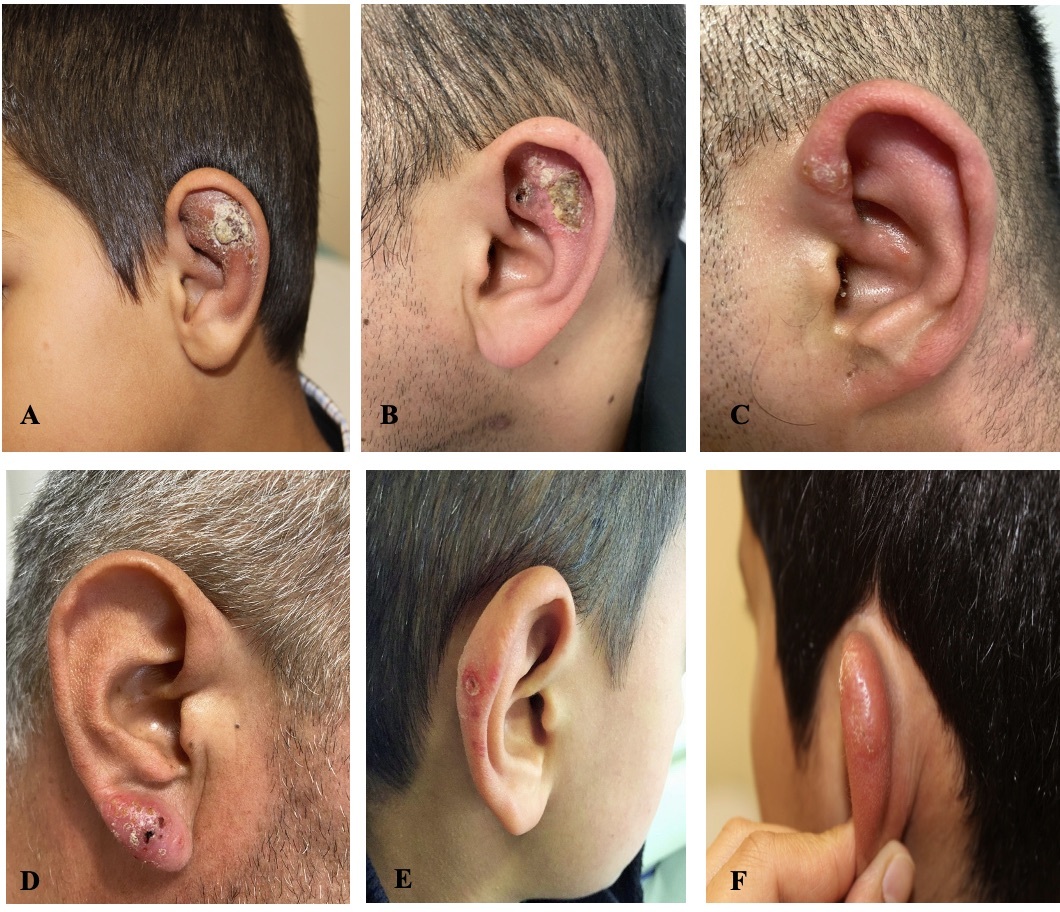 Büyütmek İçin Tıklayın |
Figure 1: A) Crusted and erythematous plaque lesion, B) Crusted and erythematous plaque lesion, C) Crusted papular lesion, D) Erythematous plaque lesion, E) Erythematous telangiectatic patch, F) Keloidal lesion. |
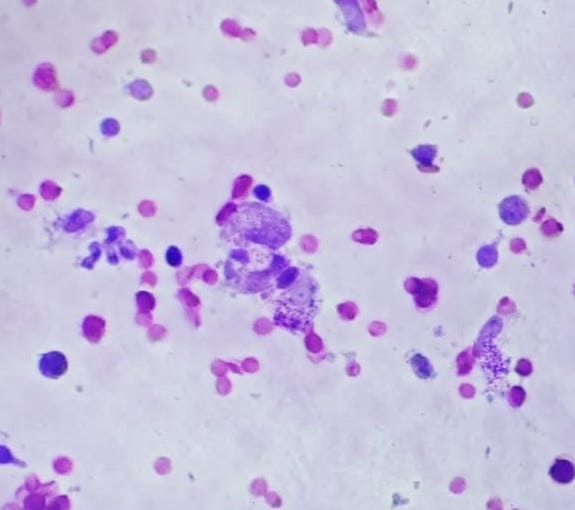 Büyütmek İçin Tıklayın |
Figure 2: Amastigotes are seen inside and outside of macrophages cells of CL lesion smears stained with Giemsa (Giemsa x40) |
Discussion
In Türkiye, a country endemic for CL, the province of Şanlıurfa is a hyperendemic region with the highest number of reported cases[3,6]. Cutaneous leishmaniasis can be seen in all age groups, but it is most commonly observed in children and adolescents[3-6]. In the present study, 58% (7/12) of the patients were under 18 years old.Cutaneous leishmaniasis is reported at a higher rate in women in endemic regions. Previous studies in our country have also shown a female predominance[1]. Aksoy et al. reported that of 8786 pediatric patients, 4050 (46.1%) were male and 4736 (53.9%) were female[6]. However, studies in other endemic countries like Iran and Tunisia with pediatric patients have reported a male predominance[15,16]. In the present study, all patients (100%) were male. This may be related to the practice of young girls wearing headscarves from an early age in the region and the freedom of boys to be outdoors during the hours when most bites occur.
In the present study, the average duration of lesions was 4.6±2.8 months. Aksoy et al. reported the average duration of lesions as 8.58 weeks[6]. The longer duration of illness in the present study could be related to the rarity of auricular involvement in CL, leading to a delayed diagnosis. The average lesion size in the present study was 3.4±2.3 cm, while Aksoy et al. reported an average lesion diameter of 12.77±0.11 mm[6]. The larger size of lesions in the present study could be due to the delayed diagnosis, allowing lesions to grow larger over time.
Cutaneous leishmaniasis lesions usually arise from bites of infected female sandflies, particularly in body areas not covered by clothing[6,12]. The head and neck region is the most affected area. In Türkiye, studies have reported head and neck involvement rates of 60.7% and 57.3%, respectively[12,13]. Similar results were observed in studies focusing solely on pediatric populations in our country and Iran[5,6,14,15]. When the head and neck region is examined by compartments, the cheek is the most commonly affected site[5,13,14,16,17]. Studies have reported either no ear involvement or ear involvement rates of 6.6%, 4.6%, and 1%[5,13,17]. The auricle anatomically consists of three parts: helix, antihelix, and lobule, and case reports in the literature have described involvement of each part separately[18-21]. In the present cases, helix involvement was found in 6 (50%) patients, antihelix in 4 (33%) patients, and lobule in 2 (17%) patients. The high frequency of helix involvement could be related to its position and surface area making it more susceptible to bites.
While ear leishmaniasis is rare in the Old World, the New World has a type known as "chiclero ulcer," most commonly affecting the pinna. It is typically seen among forest workers (chicleros) in Mexico and Central America, where Leishmania mexicana is usually the causative agent[8]. An epidemiological study in Mexico found the annual incidence of the disease to be 508 cases per 100,000 individuals[18].
The first step in diagnosing CL is clinical suspicion. While typical lesions in endemic areas easily bring CL to mind, in non-endemic areas, a high index of suspicion is required[1,2]. Laboratory methods are then needed to confirm the diagnosis. Due to its high specificity, parasitological diagnosis is still considered the gold standard. Cutaneous smear, culture, incisional biopsy, and PCR are methods aimed at demonstrating the parasite. Among these, cutaneous smear is a valuable method, especially in endemic areas, because it is cheap, quick, and easy to apply[22-25]. In the present patients, we confirmed the clinical diagnosis by observing amastigotes using the cutaneous smear method.
Cutaneous leishmaniasis lesions spontaneously heal, leaving an atrophic scar at the site. However, treatment is recommended to hasten healing, prevent unsightly scarring, prevent functional impairment, avoid relapses, prevent mucosal involvement via local spread, and remove the patient from being a disease reservoir. In treatment, antifungal agents like pentavalent antimony compounds, amphotericin-B, ketoconazole, fluconazole, itraconazole, terbinafine, pentamidine, miltefosine, paromomycin, and allopurinol, as well as physical methods like cryotherapy, thermotherapy, and photodynamic therapy can be used[1,2,25,26]. Pentavalent antimony compounds (MA, sodium stibogluconate) continue to be the first treatment choice in most countries. However, the choice of appropriate treatment often depends on the clinician's experience, patient preferences, and cost-effectiveness considerations for the patient and/or the health system[25]. We preferred pentavalent antimony compounds in all of the present cases, patients, not only to prevent the development of ugly cicatrix, destructions and dysfunction by getting a rapid response, but also because they can be obtained free of charge from the Oriental Furuncle Diagnosis and Treatment Center in our province.
These drugs can be administered both intraleisonally and systemically. Systemic therapy is recommended in chronic, recurrent, or relapsing lesions, lesions over 5 cm, inflammatory lesions, more than five lesions, lesions in locations that can cause functional impairment (over joints, eyelids, lips, etc.), mucosal involvement, proximity to mucosa and cartilage, nodular lymphangitis, immunocompromised states, and in cases unresponsive to local treatments[26].
It was choose systemic therapy for all the present cases, patients due to the risk of cartilage involvement and functional impairment in ear lesions.
According to WHO recommendations, systemic pentavalent antimony treatment for 20 days at a dose of 10-20 mg/kg/day is usually sufficient[25]. In a Tunisian pediatric series, systemic antimony treatment for 7-15 days at a dose of 20 mg/kg/day was successfully used in pediatric patients with more than five lesions or those with lesions close to the cartilage who did not respond to IL antimony treatment[16]. Intralesional treatments can also be used in patients where systemic antileishmanial treatments cannot be used due to contraindications[2,7] Yaghoobi et al successfully treated a patient with auricular leishmaniasis with intralesional meglumine antimoniate (27. Diociaiuti et al. successfully treated a pediatric patient with auricular leishmaniasis with intralesional liposomal amphotericin B[28]. We administered systemic MA treatment at a dose of 20 mg/kg/day for 20 days to all the present patients and achieved clinical improvement.
Conclusion
In CL, auricular involvement should be kept in mind, and the diagnosis should be confirmed by microscopic examination. Systemic MA treatment should be considered in patients with auricular involvement.Conflicts of interest: There are no conflicts of interest
Financial support: There is no financial support
Reference
1) An I, Aksoy M, Ozturk M, Ayhan E, Erat T, Yentur Doni N, Guldur ME. Atypical and unusual morphological variants of cutaneous leishmaniasis. Int J Clin Pract. 2021 Mar;75(3):e13730. [ Özet ]
2) Gurel MS, Tekin B, Uzun S. Cutaneous leishmaniasis: A great imitator. Clin Dermatol. 2020;38(2):140-151. [ Özet ]
3) Gürel MS, Yeşilova Y, Olgen MK, Ozbel Y. Türkiye'de Kutanöz Leishmaniasisin Durumu [Cutaneous leishmaniasis in Turkey]. Turkiye Parazitol Derg. 2012;36(2):121-9. Turkish. [ Özet ]
4) Inci R, Ozturk P, Mulayim MK, Ozyurt K, Alatas ET, Inci MF. Effect of the Syrian Civil War on Prevalence of Cutaneous Leishmaniasis in Southeastern Anatolia, Turkey. Med Sci Monit. 2015 20;21:2100-4. [ Özet ]
5) Kaya OM, Serarslan G, Dirican E. Evaluation of clinical and demographic characteristics of Turkish and Syrian pediatric cutaneous leishmaniasis patients from Hatay, Turkey after the Syrian civil war. Postepy Dermatol Alergol. 2020;37(2):229-233. [ Özet ]
6) Aksoy M, Doni N, Ozkul HU, Yesilova Y, Ardic N, Yesilova A, Ahn-Jarvis J, Oghumu S, Terrazas C, Satoskar AR. Pediatric Cutaneous Leishmaniasis in an Endemic Region in Turkey: A Retrospective Analysis of 8786 Cases during 1998-2014. PLoS Negl Trop Dis. 2016 Jul 14;10(7):e0004835. [ Özet ]
7) Uzun S, Gürel MS, Durdu M, Akyol M, Fettahlıoğlu Karaman B, Aksoy M, Aytekin S, Borlu M, İnan Doğan E, Doğramacı ÇA, Kapıcıoğlu Y, Akman-Karakaş A, Kaya TI, Mülayim MK, Özbel Y, Özensoy Töz S, Özgöztaşı O, Yeşilova Y, Harman M. Clinical practice guidelines for the diagnosis and treatment of cutaneous leishmaniasis in Turkey. Int J Dermatol. 2018 Aug;57(8):973-982. [ Özet ]
8) Martinelli C, Giorgini S, Minu MB, Orsi A, Leoncini F. Cutaneous leishmaniasis: an atypical case. Int J Dermatol. 2005;44(1):38-40. [ Özet ]
9) Robati RM, Qeisari M, Saeedi M, Karimi M. Auricular enlargement: an atypical presentation of old world cutaneous leishmaniasis. Indian J Dermatol. 2011;56(4):428-9. [ Özet ]
10) Tarkan Ö, Çetık F, Uzun S. Auricular cutaneous leishmaniasis mimicking neoplastic disease. J Laryngol Otol. 2012;126(8):821-4. [ Özet ]
11) Sabri A, Khatib L, Kanj-Sharara S, Husseini ST, Nuwayri-Salti N, Semaan R, Rameh C. Leishmaniasis of the auricle mimicking carcinoma. Am J Otolaryngol. 2009;30(4):285-7. [ Özet ]
12) Turhanoglu M, Alp Erdal S, Bayindir Bilman F. Diyarbakır Eğitim ve Araştırma Hastanesinde dokuz yıllık kutanöz leyşmanyazis olgularının değerlendirilmesi [A nine-year evaluation of cutaneous leishmaniasis patients in Diyarbakir Training and Research Hospital, Turkey]. Mikrobiyol Bul. 2014 Apr;48(2):335-40. Turkish. [ Özet ]
13) Gurel MS, Ulukanligil M, Ozbilge H. Cutaneous leishmaniasis in Sanliurfa: epidemiologic and clinical features of the last four years (1997-2000). Int J Dermatol. 2002;41(1):32-7. [ Özet ]
14) Layegh P, Moghiman T, Ahmadian Hoseini SA. Children and cutaneous leishmaniasis: a clinical report and review. J Infect Dev Ctries. 2013;7(8):614-7. [ Özet ]
15) Talari SA, Talaei R, Shajari G, Vakili Z, Taghaviardakani A. Childhood cutaneous leishmaniasis: report of 117 cases from Iran. Korean J Parasitol. 2006;44(4):355-60. [ Özet ]
16) Kharfi M, Benmously R, El Fekih N, Daoud M, Fitouri Z, Mokhtar I, Ben Becher S, Kamoun MR. Childhood leishmaniasis: report of 106 cases. Dermatol Online J. 2004 Oct 15;10(2):6. [ Özet ]
17) Bari AU. Childhood cutaneous leishmaniasis. J Clin Diagn Res. 2008;2(4):973?978.
18) Andrade-Narváez FJ, Simmonds-Díaz E, Rico-Aguilar S, Andrade-Narvéez M, Palomo-Cetina A, Canto-Lara SB, García-Miss MR, Madera-Sevilla M, Albertos-Alpuche N. Incidence of localized cutaneous leishmaniasis (chiclero's ulcer) in Mexico. Trans R Soc Trop Med Hyg. 1990 Mar-Apr;84(2):219-20. [ Özet ]
19) Abdalla N. Ambiguous skin ulcer on the ear pinna. Trop Doct. 2019;49(2):141-143. [ Özet ]
20) Adriano AL, Leal PA, Breckenfeld MP, Costa Idos S, Almeida C, Sousa AR. American tegumentary leishmaniasis: an uncommon clinical and histopathological presentation. An Bras Dermatol. 2013;88(2):260-2. [ Özet ]
21) Yaghoobi R, Pazyar N, Rafiei A, Saki N. Auricular cutaneous leishmaniasis: An unusual presentation of Leishmania major from Southwest Iran. Indian J Dermatol Venereol Leprol. 2023:1-2. [ Özet ]
22) Shirian S, Oryan A, Hatam GR, Panahi S, Daneshbod Y. Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch Pathol Lab Med. 2014;138(2):235-40. [ Özet ]
23) de Vries HJ, Reedijk SH, Schallig HD. Cutaneous leishmaniasis: recent developments in diagnosis and management. Am J Clin Dermatol. 2015;16(2):99-109. [ Özet ]
24) Sousa AQ, Pompeu MM, Frutuoso MS, Lima JW, Tinel JM, Pearson RD. Press imprint smear: a rapid, simple, and cheap method for the diagnosis of cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. Am J Trop Med Hyg. 2014;91(5):905-7. [ Özet ]
25) Minoider P, Parola P. Cutaneous leishmaniasis teratment. Travel medicine and infectious disease 2007;5(3):150-8. [ Özet ]
26) Sema Aytekin. Treatment Approaches for Cutaneous Leishmaniasis. Turkderm-Turk Arch Dermatol Venereol. 2009; 43(2): 44-47.
27) Yaghoobi R, Pazyar N, Rafiei A, Saki N. Auricular cutaneous leishmaniasis: An unusual presentation of Leishmania major from Southwest Iran. Indian J Dermatol Venereol Leprol. 2023:1-2. [ Özet ]
28) Diociaiuti A, Giancristoforo S, Calò Carducci FI, Bracaglia C, Boni A, Pane S, Onetti Muda A, De Benedetti F, Putignani L, El Hachem M. Auricular leishmaniasis in a child successfully treated with intralesional amphotericin B. Pediatr Dermatol. 2022;39(5):832-833. [ Özet ]




