COPEPTIN AND CAROTID INTIMA-MEDIA THICKNESS AS PREDICTOR BIOMARKERS OF EARLY CARDIOVASCULAR DISEASE IN SEVERE NASAL SEPTUM DEVIATED PATIENTS
2Ahi Evran Üniversitesi Tıp Fakültesi, Radyoloji, Kırşehir, Turkey
3Ahi Evran Üniversitesi Tıp Fakültesi, Biyokimya, Kırşehir, Turkey
4Ahi Evran Üniversitesi Tıp Fakültesi, Kardiyoloji, Kırşehir, Turkey
5Sağlık Bilimleri Üniversitesi Bilkent Şehir Hastanesi, Kulak Burun Boğaz, Ankara, Turkey
Summary
Objective: In our study, we aimed to analyse the carotid intima-media thickness (CIMT), systemic cardiac failure parameters, and serum copeptin concentrations in patients with severe nasal septum deviation (NSD) causing severe nasal obstruction and evaluating the diagnostic value of these parameters for the early onset of cardiovascular diseases in these patients.Methods: The sample involved 57 patients with severe NSD and 50 individuals in the control group. The diagnosis of severe NSD was made following nasal endoscopic examination. CIMT and cardiac failure parameters were evaluated with carotid ultrasonography and echocardiography. Copeptin levels in the sera of the patients were examined with the enzyme-linked immunosorbent assay method (ELISA).
Results: Right and left CIMT values, serum copeptin measurements, and log (serum copeptin level) values were significantly higher in the patient group in comparison to the control group (p<0.001 for all). In the results of the univariate analysis, the log serum copeptin and right and left carotid intima thickness variables were significantly associated with severe NSD (p<0.001). The multivariate analysis results demonstrated a significant relationship of only log serum copeptin with NSD (p=0.015). The ROC (receiver operating characteristic) curve examinations of the log serum copeptin values of the patients with severe NSD revealed an AUC (area under the curve) value of 0.72. The specificity of this measure was 86%, and its sensitivity was 50.9%.
Conclusion: When used together, serum copeptin values and CIMT measurements can serve as biomarkers for the early monitoring of cardiovascular diseases in patients with severe NSD causing complete nasal congestion.
Introduction
Nasal septum deviation (NSD) is defined as complex curvatures and deflections of the cartilage and bone septum, which prevent nasal air flow [1,2]. NSD is a prevalent source of nasal obstruction cases [2]. It is the primary cause of upper respiratory tract obstruction [3,4].Nasal obstruction contributes to the pathogenesis of obstructive sleep apnea (OSA) [5-7]. Approximately 45% of OSA patients have symptoms of nasal blockage and/or obstruction [5,6]. Severe near total nasal obstruction can result in sleep-disordered breathing [7].
When there is an almost complete obstruction of the nasal airway by NSD, hypoxia observed as a result of nasal obstruction is not rare [2].
In the early phase of atherosclerosis, a higher value of intima-media thickness is found in the arterial wall. This is observed in both the coronary vascular bed and the peripheral arteries. Carotid artery intima-media thickness (CIMT), which is an early sign of atherosclerosis, is a useful marker of subclinical atherosclerosis. Carotid atherosclerosis development in OSA patients may be mainly explained by inflammation, intermittent hypoxia, and recurrent arousal. According to previous research, OSA patients are expected to have significantly higher CIMT values, which constitute evidence for predicting cardiovascular risk in OSA patients [8-10]. The evaluation of CIMT using carotid artery ultrasonography is a non-invasive technique to identify atherosclerosis at its inchoative stages [8-10].
Severe NSD cases that cause total or partial upper airway obstruction during sleep may cause hypoxia and oxidative stress, similar to pharyngeal or hypopharyngeal collapse. Ultimately, loss of endothelial function develops, leading to atherosclerosis, cardiovascular mortality, and morbidity [2,7,9-11].
In recent years, arginine vasopressin (AVP) and copeptin have gained importance due to their diagnostic and prognostic value in various diseases. Copeptin represents vasopressin levels and is more stable in the plasma and serum due to its structural properties. Copeptin was used instead of AVP in clinical studies [12]. Copeptin concentrations may show not only an inflammatory cytokine response but also hemodynamic and osmoregulatory disorders [13].
High plasma copeptin concentrations are related to an increased likelihood of heart failure and are a significant indicator of mortality. Among coronary heart disease patients or patients suffering from chronic stable heart failure patients, elevated copeptin concentrations have been associated with poor clinical outcomes and unfavorable prognostic factors [14]. Copeptin can be a practical indicator to prevent and treat cardiac failure. Çınarka et al. observed higher copeptin levels than normal in OSA patients and reported that this increase was a significant predictor of cardiovascular complications in OSA patients [12].
We hypothesized that chronic hypoxia, hypercarbia, and free oxygen radicals that are observed in patients with severe NSD may trigger oxidative stress and cause cardiovascular morbidity and mortality just as in OSA patients [2,14,15]. Our study aimed to evaluate CIMT, systemic cardiac failure parameters, and serum copeptin levels in patients with severe NSD.
Methods
The study received the approval of the Faculty of Medicine Clinical Research Ethics Board at Ahi Evran University (decision No. 2019-02/19). The study was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patients who agreed to take part in the study.The study was performed between January 2019 and May 2020 at the Cardiology, Radiology, and Otorhinolaryngology departments of Ahi Evran University Medical Faculty Research and Training Hospital.
Complete otolaryngologic examinations were conducted in all participants. The diagnosis of NSD was made by an otorhinolaryngologist according to the clinical presence of nasal obstruction complaints and determination of NSD, by examining the patient with both anterior rhinoscopy and nasal endoscopy, using 0-degree telescopes.
The degree of NSD was evaluated with the Dreher classification (deviation, 0:none, 1:mild, 2: moderate, and 3:severe) [16]. Fifty-seven patients diagnosed with symptomatic degree 3 severe, prominent C- or S-shaped marked NSD causing near total nasal obstruction were included in the study during this period [16,17].
Nasal endoscopic examinations, paranasal sinus computed tomography imaging processes, as well as fiberoptic nasal and nasopharyngeal examinations were carried out to eliminate other potential nasal anomalies leading to upper respiratory tract obstruction. The study excluded individuals who had obstructive inferior nasal concha hypertrophy, sinusitis, concha bullosa, allergic rhinitis, nasal tumors, nasal polyposis, velopharyngeal and hypopharyngeal obstruction by 50% or more, or Grade 3 - 4 tonsil hypertrophy according to the Brodsky scale [18]. Patients who mentioned tobacco, medication, or alcohol intake were excluded. Patients accepted as obese based on their BMI were excluded. Hypertension patients, patients with heart failure, those with coronary artery disease, those with chronic pulmonary diseases, valvular heart diseases, cardiac arrhythmia cases, or conduction anomalies, and those using pacemakers were also not included in the sample.
Other exclusion criteria were medical records of any allergy, hyperlipidemia, atherosclerotic heart disease, bronchial asthma, diabetes mellitus, malignancies, hypertension, or severe systemic diseases. Individuals who indicated previous sinonasal surgical operations or any chronic disease which could destroy nasal anatomical structures were not enrolled.
The oxygen saturation rates in the peripheral blood of all patients (SpO2), their heart rates, and their diastolic blood pressure (DBP)and systolic blood pressure (SBP) values were recorded based on measurements made using a pulse oximeter and blood pressure measurement devices in the same room prior to septal surgery. The patients" weight and height measurements were kept in the records. The unit of the body mass index (BMI) was the ratio of the patient's weight in kilograms to the square of their height in meters (kg/m2). Furthermore, overnight pulse oximetry was performed in sleep in each patient keep a record of their oxygen saturation levels (SpO2) with a digital pulse oximeter. The lower limit of saturation was set as 94%, and lower values were considered to indicate hypoxemia [19].
NOSE scale
The nasal obstruction levels of the participants were identified with the Nose Obstruction Symptom Evaluation (NOSE) Scale [20]. Individuals who scored 75-100 on NOSE were included. The NOSE scale consists of 5 categories: i) nasal congestion, ii) partial or complete nasal obstruction, iii) difficulty in breathing through the nose, iv) trouble sleeping, and v) difficulty getting adequate air through the nose on exertion. It is a 5-point Likert-type scale where each item is scored from 0 indicating no problem to 4 indicating a severe problem, and the minimum and maximum scores of the scale are 0 and 20. It is recommended to multiply the respondent's score by 5 as the quality of life of a respondent is usually evaluated between 0% and 100% in other relevant scales.
Epworth Sleepiness Scale
The Epworth Sleepiness Scale (ESS) is a valid measurement instrument for excessive daytime dysfunction associated with sleepiness [21]. Higher ESS scores show that the respondent has a higher risk of OSA and needs to be directed to a specialist for additional examination.
The scales and questionnaires were completed by all patients, including ESS before septoplasty.
Carotid ultrasonography and echocardiography
CIMT and ejection fraction values were evaluated by carotid ultrasonography and echocardiography in the Cardiology and Radiology Departments of Ahi Evran University Medical Faculty Research and Training Hospital.
Evaluation of carotid intima-media thickness
The carotid arteries of the patients were examined with high-resolution B-Mode ultrasonography (Aplio 500, Toshiba, Japan) with a 4?11 MHz linear transducer. Carotid artery imaging was performed when the patient was in the supine position with their neck rotated 20° in the direction opposite to the site of examination. CIMT was measured over three positions including both of the contralateral common carotid arteries, the bifurcation region, and the first 2-cm part of the internal carotid artery; only the farther wall of the carotid artery was evaluated. The CIMT measurements were made by a longitudinal examination of the distance defined between the vascular lumen echogenicity and media/adventitia echogenicity. The CIMT values to be used were calculated from the averages of three measurements made from both carotid arteries.
Echocardiography
The transthoracic echocardiography imaging processes of all patients were carried out with a 2.5 MHz probe and the Vivid 5S General Electric Medical System (Horten, Norway) under monitoring by electrocardiography (ECG), when the patients were put into the left lateral decubitus position. All conventional echocardiographic evaluations were made in compliance with the standard guidelines published by the American Society of Echocardiography [22].
All measurement results were saved to a digital workstation system (Echopac Workstation; Vingmend Ultrasound GE) for the offline analyses. The Simpson method was used to measure the left ventricular ejection fraction (LVEF). In M-mode echocardiography, the left ventricular end-systolic diameter (LVESD), the left ventricular end-diastolic diameter (LVEDD), the left atrial (LA) diameter, and the interventricular septum diastolic (IVSd) thickness parameters were recorded, in parasternal long-axis views. In all patients, apical four-chamber images were utilized to determine the right atrial end-diastolic (RAED) and right ventricular end diastolic (RVED) diameter values. Pulmonary arterial systolic pressure (PASP) was determined based on the simplified form of Bernoulli's equation according to the tricuspid flow velocity, and the addition of the right atrial pressure (mean 5 mmHg) was made by estimation according to the respiratory collapse of the inferior vena cava (IVC). Systolic (Sm), early diastolic (Em), and late diastolic (Am) tissue Doppler analyses were conducted on apical four-chamber views via the activation of the velocity-time integral (TVI) setting of the device.
Laboratory analysis-measurement of serum copeptin levels
Venous blood was drawn by venipuncture and stored in serum gel tubes. These tubes were centrifuged for 10 minutes at 1500xg. The separated serum was packed and stored at ?80°C until being analyzed. The blood samples were thawed at room temperature prior to the analyses.
Serum copeptin levels were found by using the enzyme-linked immunosorbent assay (ELISA) technique with a commercial ELISA Kit (Human CCP, Elabscience, Houston, USA). The sensitivity of the system was calculated to be 18.75 pg/mL, and the coefficient of variation value was found as <10%.
Statistical analysis
The statistical analyses of the data were performed on the SPSS program (Version 24.0, SPSS Inc., Chicago, IL, USA. The descriptive statistics of the data are expressed as mean ± SD, median (interquartile range), or frequency (%) values. Normal distribution assumptions were tested with the Kolmogorov-Smirnov and Skewness-Kurtosis tests. The significance levels of the differences between the patient group and the control group were analyzed with independent-samples t-test for the variables that were distributed normally. The comparisons of the categorical variables were made using the Chi-squared test. p<0.05 was determined as the level of statistical significance. Spearman's correlation coefficients were tested by measuring the associations between the variables of interest. ROC (Receiver Operating Characteristic) curve analyses were employed to analyze log (serum copeptin level) and serum copeptin levels as a potential diagnostic test.
Results
This study included a total of 107 individuals, 50 in the healthy control group and 57 in the patient group. The demographic data and blood pressure levels of the participants are presented in Table 1. The mean age values of the participants were found as 28.8±8.0 and 27.3± 9.9 in the control and patient groups, respectively. There were 25 male participants (50.0%) in the control group and 29 male participants (50.9%) in the patient group. The two groups were not significantly different in terms of their age, sex, BMI, systolic blood pressure, or diastolic blood pressure values.Table 1: Baseline characteristics of study subjects
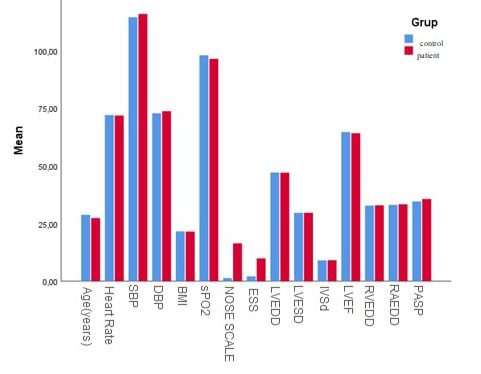
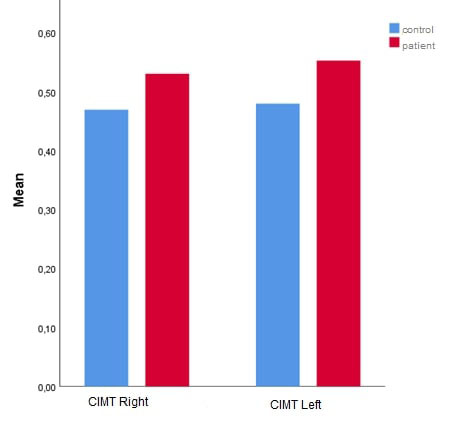
The clinical parameters of the control and patient groups are listed in Table 2. Left and right CIMT, SpO2, NOSE scale scores, ESS scores, and PASP levels were significantly higher in the patient group compared to the healthy control group (p<0.001, Figure 1, Figure 2, and Figure 3).
 Büyütmek İçin Tıklayın |
Figure 1: Comparison of baseline characteristics, echocardiographic parameters in patientswith NSD and control subjects Abbreviation: NSD, nasal septum deviation |
 Büyütmek İçin Tıklayın |
Figure 2: Comparison of demographic characteristics, echocardiographic parameters, and CIMT levels in patients with NSD and control subjects Abbreviation: CIMT, Carotid artery intima-media thickness; NSD, nasal septum deviation |
 Büyütmek İçin Tıklayın |
Figure 3: Comparison of CIMT right and left between patients with severe NSD and healthy control group. Abbreviation: CIMT, Carotid artery intima-media thickness; NSD, nasal septum deviation |
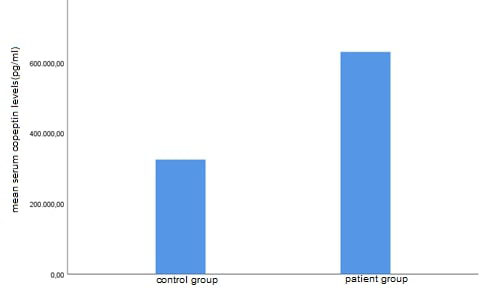
Since the distribution range of the serum copeptin level values was very wide, the analysis was continued using the logarithm of these values. Serum copeptin measurements and log (serum copeptin level) values were significantly elevated among the participants in the patient group in comparison to those in the control group (p<0.001, Figure 4) (Table 3).
 Büyütmek İçin Tıklayın |
Figure 4: Comparison of copeptin levels between patients with severe NSD and healthy control group. Abbreviation: NSD, nasal septum deviation |
Table 3: Comparison of Copeptin levels of groups
The variables (CIMT, copeptin) that were evaluated in terms of "disease risk" are summarized in Table 4. First, each variable was modeled alone, as shown in Table 4. The results are given under the title of univariate analysis. In the univariate analysis, the log serum copeptin and right and left CIMT variables were found to be significantly related to severe NSD. In the multivariate analysis, the enter logistic regression analysis technique was used. Three variables were modeled together. Although the right and left CIMT variables were insignificant because they were confounding factors, these variables were left in the model. As seen in Table 4, when a person's log serum copeptin level increased by one unit, their risk of developing the disease increased by 6.88 times.
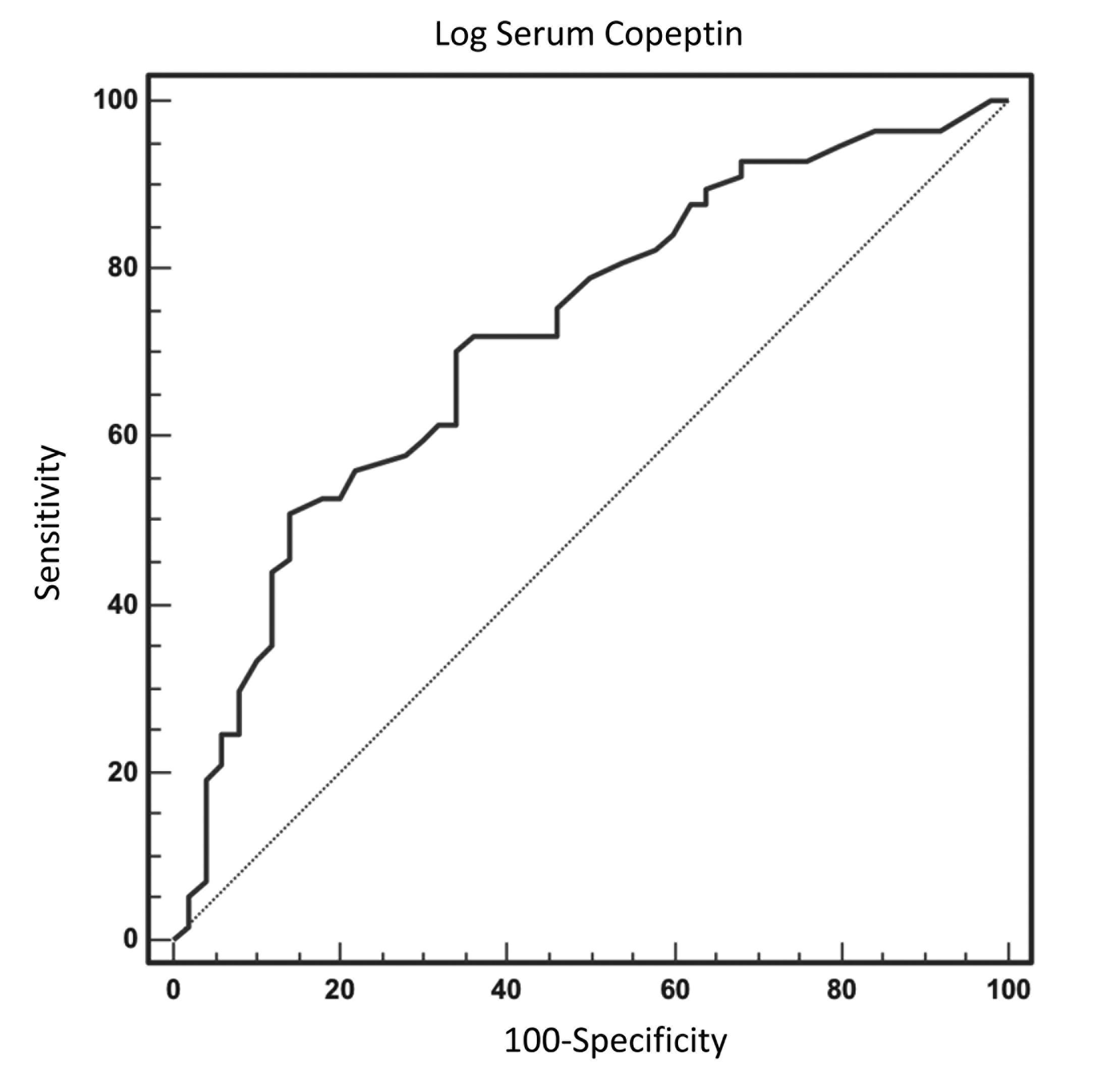
Log serum copeptin levels were evaluated by ROC analysis to determine whether this value could be used as a diagnostic parameter. The details of the ROC analysis are given in Table 5. According to the ROC curve analysis of the log serum copeptin values in the NSD patients, an AUC value of 0.72 was obtained. In this test, the specificity value was found as 86%, and sensitivity was 50.9%. The optimal cutoff value was 2.61. The ROC curve of the log serum copeptin values for the diagnosis of NSD is shown in Figure 5.
Table 5: ROC analysis results for Log serum copeptin levels
 Büyütmek İçin Tıklayın |
Figure 5: ROC curve for log serum copeptin levels Abbreviation: ROC, receiver operating characteristic |
The results of the correlation analyses between different parameters in the patient group are presented in Table 6. For the patient group in this study, the variable of patient age showed a significant correlation the right-hand-side measurement of CIMT (p<0.05). This relationship was a positive relationship with a coefficient of 28.3%. In other words, as people got older, the value of right CIMT also rose.
A statistically significant correlation was identified between SpO2 and serum copeptin levels (p<0.05). This relationship was a negative relationship with a coefficient of 35.9%. That is, as the SpO2 levels rose, the serum copeptin levels decreased. The correlation coefficients of the binary measurements that had significant relationships with each other are given in Table 6.
Table 6: The results of the correlation analysis between different parameters in patient group
The association of serum copeptin measurements and left and right CIMT values was analyzed. The patient group did not show a significant relationship of serum copeptin measurements with CIMT values. A correlation coefficient of r=0.248 was determined for the relationship of serum copeptin with right CIMT, whereas the correlation coefficient for the relationship of serum copeptin with left CIMT was r=0.162 (Table 6).
Considering the other coefficients, no statistically significant relationship was found in these binary measurements (p>0.05).
Discussion
In this study, we determined higher serum copeptin concentrations in the severe NSD patients than in the healthy controls. Additionally, CIMT was also higher in the patients with severe NSD.Cardiovascular complications of NSD are due to chronic hypoxia and hypercarbia. Hypercapnia and hypoxia originating from obstructive hypoventilation end in respiratory acidosis, which then causes pulmonary arterial vasoconstriction, higher right ventricular effort, and cardiac hypertrophy. The overall impact of chronic airway obstruction is prolonged pulmonary arterial hypertension, right ventricular failure, and cor pulmonale [1].
Previous studies have shown that severe NSD results in chronic alveolar hypoxia and hypercapnia, causing PHT and RV failure [14,15,23]. In routine clinical operations, RV failure is mostly evaluated based on PASP. In this study, the mean preoperative PASP values indicated mild PHT in the sample. The findings obtained in this study were in agreement with other findings reported in previous research [14,23].
At room temperature, the SpO2 level of a healthy person is around 97% [14]. In the sample of our study, the mean SpO2 values of participants in the patient group decreased overnight in comparison to the control group. Simşek et al. demonstrated that patients with NSD experienced oxygen desaturation, and after septoplasty, these desaturation problems significantly improved [14]. Armengot et al. evaluated patients who were treated for epistaxis with the nasal packing method, and they found that 92.5% of the patients displayed lower oxygen saturation levels, and 47% displayed severe desaturation [24].
Many pathophysiologic mechanisms have been described to clarify the effects of nasal obstruction on sleep-disordered breathing. According to the Starling resistor model, apnea can occur when nasal obstruction generates enough negative intraluminal pressure downstream to cause the compliant soft tissues of the oropharynx to collapse. In the face of significant nasal resistance, a compensatory switch to mouth-breathing may occur. Oral breathing in sleep, however, is physiologically unfavorable and unstable, and it is associated with up to 2.5-fold higher resistance developing secondarily to the narrowing of the pharyngeal lumen and further posterior collapse of the tongue, resulting in more frequent apneic events. Additionally, bypassing the nasal airway leads to less activation of the nasal receptors and the nasal-ventilatory reflex, resulting in lowered muscle tone and ventilation secondary to decreased activation of the nasal receptors. Finally, significant nitric oxide production occurs in the nose. With mouth-breathing, the decreased nitric oxide production leads to changes in the preservation of muscle tone and alterations in spontaneous ventilation and sleep patterns [5,25].
The assessment of CIMT via carotid artery ultrasonography is a non-invasive technique to identify atherosclerosis at its inchoative stages. Earlier studies have shown a rise in CIMT in OSA patients [8-10,26,27]. Carotid atherosclerosis developments in OSA patients may be mainly explained by inflammation, intermittent hypoxia, and recurrent arousal. High-frequency cycles of hypoxia and reoxygenation raise the generation of free oxygen radicals, which are implicated for lipid peroxidation and oxidative stress. Furthermore, intermittent hypoxia exacerbates the uptake of oxidized LDL by macrophages, causing the increased generation of foam cells [8]. In patients newly diagnosed with OSA without known cardiovascular diseases, Baguet et al. measured mean nocturnal arterial oxygen saturation levels and demonstrated that the severity of oxygen desaturation can be a predictive factor of CIMT [28].
According to recent studies, VEGF can contribute to the course of atherogenesis. Hypoxia has been reported as a major stimulus that raises VEGF levels. Moreover, it has been shown that VEGF levels are elevated among OSA cases compared to control groups [10]. Oxidative stress following intermittent hypoxia in severe NSD patients with or without obstructive sleep apnea (OSA) can play a significant part in the cardiovascular disease risk of these individuals, and oxidative stress in this case is correlated with surrogate indicators of cardiovascular disease, such as CIMT [2,8]. There was no article in the literature considering the relationship between CIMT and severe NSD. Because previous studies showed severe NSD associated with OSA, hypoxia, and oxidative stress, we studied CIMT measurements using carotid artery ultrasound in severe NSD patients. In this recent study, the right and left CIMT measurements of the patients were higher than those of the healthy controls.
The serum levels of arginine vasopressin (AVP), also called antidiuretic hormone, increase in certain pathological conditions such as cardiovascular diseases [11]. AVP has low stability in the circulation. On the other hand, copeptin, a C-terminal peptide of pro-AVP, has higher stability in the circulation and can be used to monitor AVP levels using immunoassays [11,29]. In addition to cardiovascular diseases, copeptin levels were found to be higher in lower respiratory tract infections and chronic obstructive pulmonary disease, as well as in OSA patients, and a higher amount of circulating copeptin has been reported as an indicator for poor prognosis or long-term clinical failure [30-32]. Oxidative stress and hypoxia may lead to the release of antidiuretic hormone and increased copeptin concentrations [30]. Hypoxia induced by OSA, hemodynamic factors, oxidative stress, vascular endothelial dysfunction, sympathetic activation, metabolic dysregulation, in addition to inflammation, can result in heightened concentrations of copeptin in the circulation, as well as pro-atrial natriuretic peptide (pro-ANP), and pro-adrenomedullin (pro-ADM) [12,33]. Oxidative stress and hypoxia may lead to the release of antidiuretic hormone and increased copeptin concentrations [30,33]. Therefore, relevant studies have suggested that there may be a link between oxidative stress and copeptin. In support of this idea, rats exposed to acute hypoxia conditions had increased serum levels of copeptin [30]. In a previous study, in was reported that there were hypoxia and oxidative stress in patients with severe NSD [2]. We also investigated serum copeptin concentrations in a similar group of patients. We identified elevated levels of copeptin in these severe NSD patients. This may be linked to hypoxia and oxidative stress in these patients. Besides, copeptin values were almost two-fold in comparison to the values of the participants in the control group.
Since serum copeptin values had a very wide distribution, we chose to use logarithm serum copeptin values in our further statistical analyses. Log serum copeptin values were previously used in statistical analyses that investigated the association of serum copeptin values and heart failure [13]. Through the regression analysis of the relationships between log serum copeptin, right CIMT, left CIMT, and severe NSD, the relationships of log serum copeptin, right CIMT, and left CIMT with severe NSD were shown to be significant using the univariate analysis. On the other hand, the results of the multivariate analysis indicated a relationship of only log serum copeptin with severe NSD. Based on the multivariate logistic regression analysis results, the logarithm of serum copeptin levels was an independent predictive factor of severe NSD. This statistical analysis result meant that when a person's log serum copeptin levels increased by one unit, their risk of developing the disease increased by almost seven times. These data indicated that serum copeptin measurements could have a more reliable relationship to severe NSD than CIMT values do. Since log serum copeptin values had a significant relationship to severe NSD in the multivariate regression analysis in this study, we conducted an ROC curve analysis using log serum values in the severe NSD patients to determine the potential diagnostic value of log serum copeptin values. The ROC curve analysis of the log serum copeptin values in the patient group resulted in an AUC value of 0.72. The specificity of this test was 86%, and its sensitivity was 50.9%, while the cut off value was found as 2.61. This cutoff value was a logarithmic value. Therefore, converting this value back to serum copeptin measurements may provide an idea of the minimum serum copeptin values that may be associated with severe NSD. This value approximately corresponded to a value of 407 pg/ml of serum copeptin.
Several researchers have reported relationships between sleepiness secondary to nasal septal deviation and OSA [34]. Patients who have nasal obstruction caused by septal deviation may suffer from sleep-disordered breathing, daytime dysfunction, and fatigue, which tend to have significant physical and social consequences in patients [35]. In a previous study, it was considered that NSD was significantly more frequently encountered among students who appeared sleepy (46.8%) in comparison to their attentive peers (22.8%) [34]. We used the Nasal Obstruction Symptom Evaluation Scale with the Epworth Sleepiness Scale for measuring daytime dysfunction. Similar to the literature, we also found higher ESS scores in the severe NSD patients in comparison to the control group.
To the best of our knowledge, our study is the first one in the literature to investigate the relationship between severe NSD and copeptin, carotid intima thickness, and cardiac parameters.
Limitations
A few limitations of this study should also be noted. The main limitation was that we could not include the data of the patients after septoplasty due to the difficulty of their follow-up. The other limitation was that we did not have the chance to perform polysomnography among our patients.
Conclusion
when serum copeptin measurements and CIMT values are considered together, these measurements may serve as markers to monitor cardiovascular disease risk at an early stage in patients with severe NSD.
Submission Statements
Ethics Committee Approval: This study was approved by Ethics committee of Medical Faculty of ??.. University (Approval No: 2019-02/19).
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support
Reference
1) Sagit M, Korkmaz F, Kavugudurmaz M, Somdas MA. Impact of septoplasty on mean platelet volume levels in patients with marked nasal septal deviation. J Craniofac Surg. 2012;23(4):974- 976. [ Özet ]
2) Ekinci A, Karatas D, Yetis A, Demir E, Ozcan M. The effects of septoplasty surgery on serum oxidative stress levels. European Archives of Otorhinolaryngology 2017;274 (7): 2799-2802. [ Özet ]
3) Michels Dde S, Rodrigues Ada M, Nakanishi M, Sampaio AL, Venosa AR. Nasal involvement in obstructive sleep apnea syndrome. Int J Otolaryngol. 2014;2014:717419. [ Özet ]
4) Georgalas C. The role of the nose in snoring and obstructive sleep apnoea: an update. Eur Arch Otorhinolaryngol. 2011;268(9):1365-1373. [ Özet ]
5) Mickelson SA. Nasal Surgery for Obstructive Sleep Apnea Syndrome. Otolaryngol Clin North Am. 2016;49(6):1373-1381. [ Özet ]
6) Awad MI, Kacker A. Nasal Obstruction Considerations in Sleep Apnea. Otolaryngol Clin North Am. 2018;51(5):1003-1009. [ Özet ]
7) Silvoniemi P, Suonpää J, Sipilä J, Grénman R, Erkinjuntti M. Sleep disorders in patients with severe nasal obstruction due to septal deviation. Acta Otolaryngol Suppl. 1997;529:199-201. [ Özet ]
8) Zhou M, Guo B, Wang Y, Yan D, Lin C, Shi Z. The Association Between Obstructive Sleep Apnea and Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Angiology. 2017;68(7):575-583. [ Özet ]
9) Farooqui FA, Sharma SK, Kumar A, Soneja M, Mani K, Radhakrishnan R, Farooqui N. Endothelial function and carotid intima media thic(kness in obstructive sleep apnea without comorbidity. Sleep Breath. 2017;21(1):69-76. [ Özet ]
10) Suzuki T, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Yamauchi M, Kimura H. Obstructive sleep apnea and carotid-artery intima-media thickness. Sleep. 2004;27(1):129-133. [ Özet ]
11) Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. 2017;14(2):135-150. [ Özet ]
12) Çınarka H, Kayhan S, Karataş M, Yavuz A, Gümüş A, Özyurt S, Cüre MC, Şahin Ü. Copeptin: a new predictor for severe obstructive sleep apnea. Ther Clin Risk Manag. 2015;10(11):589-594. [ Özet ]
13) Yan JJ, Lu Y, Kuai ZP, Yong YH. Predictive value of plasma copeptin level for the risk and mortality of heart failure: a meta-analysis. Journal of Cellular and Molecular Medicine 2017;21(9):1815-1825. [ Özet ]
14) Simsek Z, Simsek E. Does Nasal Surgery Affect Right Ventricular Myocardial Functions at the Tissue Level in Patients with Nasal Septum Deviation? J Clin Med. 2018;7(8):186. [ Özet ]
15) Kaya H, Kurt E, Koparal M, Tibilli H, Hosoglu Y, Kafadar S, Suner A, Türkmen S. Effect of septoplasty on left ventricular myocardial performance in patients with nasal septum deviation. Braz J Otorhinolaryngol. 2020;26(20):30151-30158. [ Özet ]
16) Dreher A, de la Chaux R, Klemens C, Werner R, Baker F, Barthlen G, Rasp G. Correlation between otorhinolaryngologic evaluation and severity of obstructive sleep apnea syndrome in snorers. Arch Otolaryngol Head Neck Surg. 2005;131(2):95-98. [ Özet ]
17) Teixeira J, Certal V, Chang ET, Camacho M. Nasal Septal Deviations: A Systematic Review of Classification Systems. Plast Surg Int. 2016;2016:7089123. [ Özet ]
18) Ceran O, Aka S, Oztemel D, Uyanik B, Ozkozaci T. The relationship of tonsillar hyperplasia and asthma in a group of asthmatic children. Int J Pediatr Otorhinolaryngol. 2004;68(6):775-778. [ Özet ]
19) Esteban-Amarilla C, Martin-Bote S, Jurado-Garcia A, Palomares-Muriana A, Feu-Collado N, Jurado-Gamez B. Usefulness of Home Overnight Pulse Oximetry in Patients with Suspected Sleep-Disordered Breathing. Can Respir J. 2020;12:1891285. [ Özet ]
20) Karahatay S, Taşlı H, Karakoç Ö, Aydın Ü, Türker T. Reliability and validity of the Turkish Nose Obstruction Symptom Evaluation (NOSE) scale. Turk J Med Sci. 2018;48(2):212-216. [ Özet ]
21) Ishii L, Godoy A, Ishman SL, Gourin CG, Ishii M. The nasal obstruction symptom evaluation survey as a screening tool for obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2011;137(2):119-23. [ Özet ]
22) Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463. [ Özet ]
23) Ozkececi G, Akci O, Bucak A, Ulu S, Yalım Z, Aycicek A, Onrat E, Avsar A. The effect of septoplasty on pulmonary artery pressure and right ventricular function in nasal septum deviation. Eur Arch Otorhinolaryngol. 2016;273(11):3747-3752. [ Özet ]
24) Armengot M, Hernández R, Miguel P, Navarro R, Basterra J. Effect of total nasal obstruction on nocturnal oxygen saturation. Am J Rhinol. 2008;22(3):325-328. [ Özet ]
25) Awad MI, Kacker A. Nasal Obstruction Considerations in Sleep Apnea. Otolaryngol Clin North Am. 2018;51(5):1003-1009. [ Özet ]
26) Polak JF, O'Leary DH. Carotid Intima-Media Thickness as Surrogate for and Predictor of CVD. Glob Heart. 2016;11(3):295-312. [ Özet ]
27) Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7(10):1025-38. [ Özet ]
28) Baguet JP, Hammer L, Lévy P, Pierre H, Launois S, Mallion JM, Pépin JL. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest 2005;128:3407-3412. [ Özet ]
29) Robertson GL, Mahr EA, Athar S, Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. Journal of Clinical Investigation 1973;52 (9):2340-2352. [ Özet ]
30) Ostergaard L, Rudiger A, Wellmann S, Gammella E, Beck-Schimmer B, Struck J, Maggiorini M, Gassmann M. Arginine-vasopressin marker copeptin is a sensitive plasma surrogate of hypoxic exposure. Hypoxia (Auckl) 2014;11(2):143-151. [ Özet ]
31) Müller B, Morgenthaler N, Stolz D, Schuetz P, Müller C, Bingisser R, Bergmann A, Tamm M. Circulating levels of copeptin, a novel biomarker, in lower respiratory tract infections. European Journal of Clinical Investigation 2007;37(2):145-152. [ Özet ]
32) Stolz D, Christ-Crain M, Morgenthaler NG, Leuppi J, Miedinger D, Bingisser R, Müller C, Struck J, Müller B, Tamm M. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest 2007;131(4):1058-1067. [ Özet ]
33) Karakioulaki M, Grendelmeier P, Strobel W, Schmid T, Jahn K, Grize L, Tamm M, Stolz D. Copeptin, pro-atrial natriuretic peptide and pro-adrenomedullin as markers of hypoxic stress in patients with obstructive sleep apnea-a prospective intervention study. Respir Res. 2021;22(1):114. [ Özet ]
34) Shirvani ME, Tayebi S, Khashayar P, Pourabbasi A. The Relationship Between Nasal Septal Deviation, Daytime Sleepiness and School Performance Among Iranian High School Students: A Pilot Cross-Sectional Study. Int J School Health. 2015;2(3): e26482.
35) Valsamidis K, Titelis K, Karkos P, Markou K, Constantinidis J, Triaridis S. Predictive factors of patients' general quality of life after nasal septoplasty. Eur Arch Otorhinolaryngol. 2019;276(2):429-438. [ Özet ]




