CORRELATION OF STERNOCLEIDOMASTOID MUSCLE THICKNESS WITH RADIOLOGICAL GRADE IN ADULTS WITH COVID-19
2Elazığ Fethi Sekin Şehir Hastanesi, Ear Nose Throat Clinic, Merkez, Turkey
3Elazığ Fethi Sekin Şehir Hastanesi, Radiology, Merkez, Turkey
Summary
Aim: Our study aimed to determine the relationship between accessory muscle thickness and radiological grade in Covid -19 disease.Material methods: 350 patients who were admitted between March 2020 and September 2020 with the pre-diagnosis or diagnosis of COVID-19. The control group included 183 patients who were evaluated for Thoracic CT for any reason and were evaluated as normal and not diagnosed with COVID-19. Images of the patients were evaluated retrospectively. Right and left sternocleidomastoid muscle (SCM) thickness measurements of all patients were measured at the level of the thyroid gland in thorax CT without contrast.
Result: Of the patients with CT involvement, 48.85% were female, and 51.14% were male. The control group consisted of 45.35% women and 54.64% men. The ages of the patients with CT involvement were 49.8 ± 12.65 (18-70), and the age of the control group was 48.9 ± 11.5 (28-85). A significant difference was found between the right and left SCM diameter changes when patients with CT involvement were compared with the control group (p: 0.025, p: 0.001, respectively). Right and left SCM diameters were significantly increased in those with consolidation (p: 0.042, p: 0.01, respectively) and positively correlated (r: 0.08, r: 0.112).
Conclusion: In our study, in patients with respiratory distress, accessory muscles are activated to support breathing, and hypertrophy developed due to overuse of the muscles was supported by the measurements made in CT sections.
Introduction
SARS-Cov-2 (COVID-19) is a coronavirus belonging to the coronaviridae family, which was first detected in Wuhan, China, at the end of 2019[1] The World Health Organization declared COVID-19 disease as a pandemic on March 11, 2020[2]. This rapidly spreading virus threatens the health of the world and increases the burden on the health systems of countries day by day. Although COVID-19 infection significantly affects the respiratory system in the acute period, it also affects the heart and circulatory system, and muscular and neurological systems[3,4,5].The most common symptoms are cough, high fever, and forced breathing. While most individuals with COVID-19 develop mild to moderate self-limiting viral illness, more than one in ten develop severe respiratory symptoms and, most commonly, a viral pneumonic process, often leading to hypoxemic respiratory failure[6]. Our knowledge of the clinical features of COVID-19 infection is limited. In this context, computed tomography (CT) imaging shows diffuse interstitial changes with ground glass-type opacities in 75% of patients hospitalized with COVID-19[7]. There is no virus-specific precaution and treatment. Symptomatic treatment is applied to patients. Respiratory muscle performance decreases in conditions such as aging, smoking, and chronic disease[8,9]. Studies with patients with moderate to severe SARS infections have clarified the musculoskeletal burden of this disease by showing skeletal muscle, neurological, bone, and joint damage[10,11].
In an acutely ill lung (e. g. ARDS), the pressure required to breathe increases further, which can increase the risk of respiratory failure[9]. The main respiratory muscle is the diaphragm. When the diaphragm is forced, the accessory inspiratory muscles (parasternal-, external intercostal-, scalene-, and sternocleidomastoid muscles) are involved[8]. Studies in patients with respiratory distress have mostly focused on the diaphragm[10]. However, the role of accessory muscles in forced breathing has been largely neglected in the literature. There are concerns about potential long-term pulmonary sequelae and related impairment in functional capacity in people recovering from COVID-19. When the respiratory need cannot be met by the respiratory muscles, respiratory muscle synergists come into play. Thus, the activity of respiratory muscle synergists increases. Also, patients choose a more comfortable position for breathing. It causes changes in the functions of involuntary muscles. In particular, the sternocleidomastoid and scalene muscles, the muscles of the shoulder girdle, undertake to support the respiratory muscles in difficult breathing. Skeletal muscle hypertrophy is defined as an increase in muscle mass and cross-sectional area at all tissue and cellular levels[11].
Overuse of muscle can cause an increase in muscle diameter. An increase in the thickening fraction of the muscle occurs as a result of the use of accessory respiratory muscles[12]. In this study, we aimed to discuss the role of respiratory muscles in respiration, and we wanted to emphasize the importance of correct respiratory rehabilitation in the early period by evaluating the diameter changes of the sternocleidomastoid muscles in patients with respiratory muscle weakness with a diagnosis of COVID-19.
Methods
The required permission from the Ministry of Health and the approval of the local Ethics Committee (Date: 05/11/2020, Number: 2020/15-14) were obtained for the present study. The study was retrospective, and there was no need for an informed consent form as there were no new or additional risks for the participants. However, the study was conducted according to the principles of the Declaration of Helsinki.
Study population and management:
Diagnostic and demographic information, laboratory values, and images of the patients were scanned retrospectively from the hospital information system (HIS). The clinical data and CT images of 350 consecutive patients who applied to the pandemic outpatient clinic with a pre- diagnosis of COVID-19 between March and September 2020 and who presented to the Physical Therapy and Rehabilitation outpatient clinic due to musculoskeletal complaints were examined. Patients with a past history of lung disease, heart disease, rheumatic disease, myositis, trauma, muscle disease, thyroid or parathyroid disease, malignancy, or steroid use were excluded from the study.
According to CORDS classification, CT Findings Related to COVID-19 criteria [17], 350 patients (171 female and 179 male) have positive findings on thoracic CT and undergoing COVID-19 treatments were selected for the study. In the same period, 183 patients (83 female and 100 male) who were evaluated as normal due to the absence of lung involvement in thoracic CT examination performed for another reason and who could not be diagnosed with COVID-19 and whose RRT-PCR test was negative were selected as controls.
In the group selected as the patient, those with significant involvement in Thoracic CT in terms of COVID-19 were evaluated. Patients with ground glass opacities (GGO) as CT signs were evaluated in the mild disease group, and patients with extensive ground-glass consolidation were evaluated in the moderate-to-severe disease group.
Image acquisition:
All imaging procedures were performed with a multi-detector sequential CT scanner (Ingenuity Elite, Philips Healthcare, Best, Netherlands). The CT protocol consisted of 120 kV-100 mA tube current, 2 mm slice thickness, and the patient in the supine position and inspiration without using contrast media.
Image analysis:
The analysis of the images was carried out using the DICOM viewer (FONET, v4.1, Ankara, TR) of the PACS system integrated into HIS. The thickness of the right and left SCM and trapezius muscles of all participants were measured by an 8-year experienced radiologist (P.G.B.) on axial thoracic CT images, where they are best seen at the level of the thyroid gland. The muscle densities in the axial sections were evaluated as the average of three measurements taken in Hounsfield units (HU) using equal ROIs (Region of Interest) from each muscle (Fig. 1).
Statistical analysis
All statistical analyzes were performed using SPSS version 25.0 (SPSS Inc, Chicago, IL). Data were expressed as mean ± standard deviation. The Chi-square test was used for gender comparison between the two groups. The normality of the distribution was assessed using the Kolmogorov-Smirnov test. According to these results, the Mann-Whitney U test and Kruskal Wallis test was used to compare the groups Tamhane's T2 test was used for Post hoc Spearman's rho correlation test was used to correlate variables in the study group.
In addition, logistic regression analysis was performed for all parameters. The p < 0.05 was considered significant.
Results
The gender distribution of all patients is shown in Table 1, and the age values of the groups are shown in Table 2. İn our study, 350 patients with thoracic CT involvement and 183 control patients with normal CT were included. There was no statistically significant difference between the groups regarding age and gender (p: 0.4, p: 0.5, respectively). There were ground-glass opacities in 254 (72.57%) patients with CT involvement and consolidation in 96 (27.42%) patients. The RRT-PCR test was positive for 254 (72.57%) COVID-19 patients.Table 1. Age and SCM diameter measurements of patients with CT involvement and control group
When the groups were compared in terms of the diameter changes of the SCM muscle, a significant increase was found in the muscle diameter of the COVID-19 patient groups with the radiological degree. (p:0,025, p: 0,001, respectively) In addition to this finding, a significant decrease was found in the density values of the SCM muscle, the radiological grade, and the muscle density of the COVID-19 patient groups. The presence of consolidation was positively associated with age (p: 0.00).
Table 3: CT Image Values of Right SCM and Left SCM Muscles
SCM diameters were significantly increased in those with consolidation (p: 0.042, p:0.01, respectively), and as seen in Figure 1.2, there was a positive correlation between CT classification grade and SCM muscle diameters. (r: 0.08, r: 120 0.112).
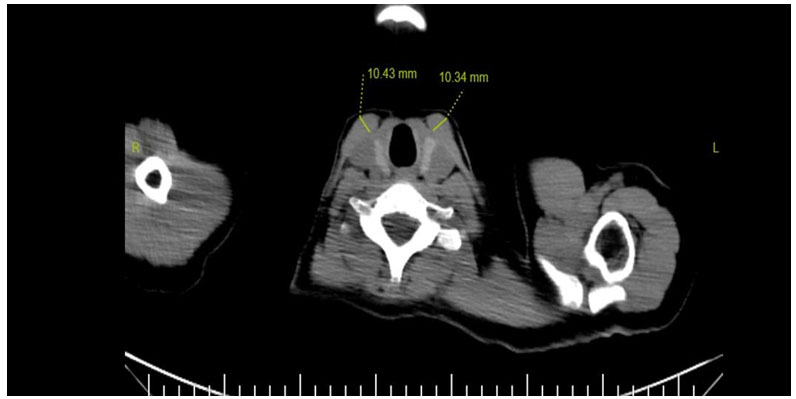 Büyütmek İçin Tıklayın |
Fig 1: SCM thickness measurement at the level of the thyroid gland in thorax CT without contrast |
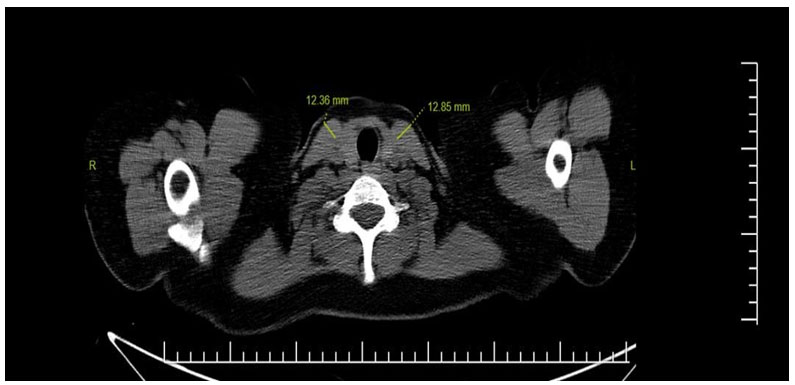 Büyütmek İçin Tıklayın |
Fig 2: SCM hypertrophy at the level of the thyroid gland in thorax CT without contrast |
Discussion
Emerging in the past decade, SARS, H1N1, Influenza A, and Middle East Respiratory Syndrome (MERS) has demonstrated the strong threat of new human diseases of global impact. In the SARS-CoV-2 outbreak (COVID-19), the number of cases continues to increase rapidly all over the World. In most cases of COVID-19, mild symptoms such as fever (80%), dry cough (56%), fatigue (22%), muscle pain (7%) can be seen as well as acute respiratory failure syndrome (ARDS)[3,13].Although diagnosis is based on a real-time reverse transcriptase-polymerase chain reaction (RRT-PCR) positivity for the presence of coronavirus, rapid and reliable testing is needed to reduce transmission and mortality. RRT-PCR results usually require 5 to 6 hours, whereas CT exam results can be obtained much faster. In addition, it remains unclear whether RRT-PCR is the gold standard and whether false positive and false negative results are common. In studies where RRT-PCR test results of the patients were taken as reference, the sensitivity, specificity, and accuracy of Thorax CT in demonstrating COVID-19 infection was found to be approximately 95-97[13,14,15]. These findings supported our idea that chest CT is useful not only for its rapid results in cases suggestive of covid-19 infection but also for the initial evaluation and rapid isolation of patients. As in recent studies, RRT-PCR positivity was detected in 72.57% of patients diagnosed with COVID-19, and thoracic CT scans of the patients were interpreted in terms of COVID-19 involvement according to the CORDS[16]. The clinical features and severity of COVID-19 disease vary greatly from asymptomatic to fatal. The most common symptoms are fever, cough, difficulty breathing, and respiratory distress. Although 81% of the cases show a mild course, severe pneumonia in 14%, respiratory failure, ARDS, and multi-organ failure develops in 5%. The main respiratory muscles are the chest and abdominal muscles, and during inspiration, the diaphragm, scalene, SCM, parasternal and intercostal muscles are activated. Deep weakness of respiratory muscles has been shown to occur in patients with respiratory failure[17,18,19] Pre-existing respiratory symptoms may progress for some time. trouble-free respiratory function due to weakened respiratory muscles, especially for the elderly and patients with pre-existing respiratory disorders (e.g. COPD, restrictive lung diseases)[20]. Patients with dyspnea try to support their breathing by increasing the use of accessory inspiratory muscles. Especially when the diaphragm function, which is the main respiratory muscle, is impaired, synergist respiratory muscles enter the circuit. The scalene muscle and the sternocleidomastoid muscle are flexors of the cervical vertebra and are effective in controlling the posture of the neck. Respiratory muscle fibers can change their properties to adapt to new requirements that may arise from physiological conditions, lung / respiratory diseases, as well as functional tasks[19]. They also work as auxiliary inspiratory muscles to aid breathing. These muscles are effective in breathing as well as functional movements of the neck[18]. Many studies have focused on diaphragm structure and function in patients with acute respiratory failure. However, there are very few reports in the literature regarding the role of accessory muscles in respiratory physiology in respiratory failure.
In our study, we aimed to evaluate the respiratory assist muscles in patients diagnosed with COVID-19 with pulmonary involvement. While CT provides useful information about the integrity and structure of the airways and lung parenchyma, it also has the potential to examine the contribution of respiratory muscles to lung function. Imaging techniques can provide information about the integrity and structure of the airways and lung parenchyma. CT can be used to examine the respiratory muscles, particularly the diaphragm[21]. Thoracic CT scans of the patients had bilateral peripheral ground-glass opacities and typical COVID-19 involvement interpreted as consolidation[7].
Respiratory muscle fibers can change their properties to adapt to new requirements that may arise from respiratory diseases or functional physiological conditions[18,22]. In particular, we evaluated the diameter change of the SCM muscle, one of the inspiratory auxiliary muscles, in the part of the image area according to the standard shooting protocol. SCM diameter increased significantly in the patient group compared to the control group. In the SCM muscle, hypertrophy may develop as a result of the increased workload under the influence of the inflammatory process. We evaluated muscle hypertrophy caused by excessive use of muscles as an increase in muscle diameter. At the same time, SCM diameter increased significantly in patients with CT consolidation. The increase in SCM muscle diameter in patients with consolidation made us think that the use of accessory muscles of the patients was activated as the workload increased while breathing. Death risk in COVID-19 increases significantly with age. Those diagnosed with COVID-19.
Case fatality rates were 0.1% in children, compared to 14.8% in the elderly in China[23]. In our patients, the presence of consolidation on CT was positively correlated with age and positively correlated (p: 0,00, r: 0,154). Pulmonary findings worsened as the patients got older.
Conclusion
In this study, it was determined that accessory respiratory muscles were activated to support respiration in patients with respiratory distress. The increase in muscle diameter due to overuse of the muscles has shown us the severity of respiratory distress caused by Covid-19. Hypertrophy and changes in density in the muscles in the early period indicate that the adipose tissue replacing healthy muscle tissue will accelerate the progression of respiratory muscle weakness in the following periods. The proportion of patients with consolidation on CT supported our view on this issue considering the rate of deterioration in respiratory functions in covid-19 patients, the importance of early rehabilitation and supporting correct respiratory muscle use becomes evident once again.
Limitations:
The limitations of our study were that it was single-centered, the number of patients was small, and we could not examine the patients. The data of our patients were evaluated retrospectively, but our knowledge about clinical information and follow-up was limited.
Conflict of interest: No conflict of interest was declared between the authors.
Statement of Contribution of Researchers: The authors declare that they contributed at all stages of the article.
Support / Statement of Acknowledgment: No financial support was received from any institution or person in this study.
Ethics Committee / Statement of Informed Consent: This study was approved by the Fırat University Clinical Research Ethics Committee (05/11/2020, Number: 2020/15-14).
Reference
1) Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X., Song J, Zhao X, Huang B,
Shi W, Ph.D., Roujian Lu, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao G D.Phil.,
andTan W for the China Novel Coronavirus Investigating and Research Teamet al. A Novel
Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33. (10.1056/NEJMoa2001017)
2) 3) Wu Y-C, Chen C-S, Chan Y-J. The outbreak of COVID-19: An overview. J Chinese Med Assoc. 2020;83(3):217-20. (10.1097/JCMA.0000000000000270, [ Özet ]
4) Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683. (10.1001/jamaneurol.2020.1127, [ Özet ]
5) Li Y, Qin L, Shi Y, Han J. The Psychological Symptoms of College Students in China during the Lockdown of the COVID-19 Epidemic. Healthcare. 2021;9(4):447. (doi: 10.3390/healthcare9040447, [ Özet ]
6) Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19). JAMA. 2020;25:782. ( 10.1001/jama.2020.12839)
7) Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chungl M Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295(3):200463. (10.1148/radiol.2020200463. Epub 2020, [ Özet ]
8) Shi Z-H, Jonkman A, Vries H, Jansen D, Ottenheijm C, Girbes A, Man A-S, ZhouJ-X, Laurent Brochard & Leo Heunks. Expiratory muscle dysfunction in critically ill patients: towards improved understanding. Intensive Care Med. 2019;45(8):1061-71.
[ Özet ], DOI: 10.1007/s00134-019-05664-4)
9) Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser L-R, Rubinstein N-A, Powers S-K, Shrager J. Rapid Disuse Atrophy of Diaphragm Fibers in Mechanically Ventilated Humans. N Engl J Med. 2008;358(13):1327?35. ( 10.1056/NEJMoa070447, [ Özet ]
10) Jonkman AH, Jansen D, Heunks LMA. Novel insights in ICU-acquired respiratory muscle dysfunction: implications for clinical care. Crit Care. 2017;21(1):64. (10.1186/s13054-017-1642-0, [ Özet ]
11) Russell B, Motlagh D, Ashley WW. Form follows function: how muscle shape is regulated by work. J Appl Physiol. 2000;88(3):1127?32. ( 10.1152/jappl.2000.88.3.1127, [ Özet ]
12) Dres M, Dubé BP, Goligher E, Vorona S, Demiri S, Morawiec E, et al. Usefulness of
Parasternal Intercostal Muscle Ultrasound during Weaning from Mechanical Ventilation. Anesthesiology. 2020;132(5):1114-25. ( 10.1097/ALN.0000000000003191, [ Özet ]
13) Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, Zeng B, Zicong Li, Li X, Li H
Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? EurJ Radiol.2020;126:108961. (10.1016/j.ejrad.2020.108961. Epub 2020 March 25, [ Özet ]
14) Lal A, Mishra AK, Sahu KK. CT chest findings in coronavirus disease-19 (COVID-19). J Formos Med Assoc. 2020;119(5):1000?1. (10.1016/j.jfma.2020.03.010)
15) Rona G, Arifoğlu M, Voyvoda N, Batırel A. Should CT be used for the diagnosis of RT?PCR?negative suspected COVID?19 patients? Clin Respir J.2021;15(5):491-8
(10.1111/crj.13332, [ Özet ]
16) Prokop M, Van Everdingen W, Van Rees Vellinga T, Quarles van Ufford H, Stöger L, Beenen L, Geurts B, Gietema H, Krdzalic J, Prokop C, Ginneken B, Brink M. COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society 1et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology. 2020;296(2):97-104. (10.1148/radiol.2020201473, [ Özet ]
17) Daniel Martin A, Smith BK, Gabrielli A. Mechanical ventilation, diaphragm weakness, and weaning: A rehabilitation perspective. Respir Physiol Neurobiol. 2013;189(2):377-83. (10.1016/j.resp.2013.05.012, [ Özet ]
18) Kang J, Jeong D-K, Choi H. The effects of breathing exercise types on respiratory muscle activity and body function in patients with mild chronic obstructive pulmonary disease. J Phys Ther Sci. 2016;28(2):500-5. (10.1589/jpts.28.500, [ Özet ]
19) Hudson AL, Gandevia SC, Butler JE. The effect of lung volume on the coordinated recruitment of scalene and sternomastoid muscles in humans. J Physiol. 2007;584(1):261-70. (10.1113/jphysiol.2007.137240, [ Özet ]
20) Van der Lee L, Hill A-M, Patman S. After-hours respiratory physiotherapy for intubated and mechanically ventilated patients with community-acquired pneumonia: An Australian perspective. Aust Crit Care. 2018;31(6):349-54. (10.1016/j.aucc.2017.10.001, [ Özet ]
21) Harlaar L, Ciet P, Van Tulder G, Pittaro A, Van Kooten HA, Van der Beek NAME, Brusse E, Wielopolski P-A, Bruijne M, Ploeg A-T, Tiddens H, Doorn P-A. Chest MRI to diagnose early diaphragmatic weakness in Pompe disease. Orphanet J Rare Dis. 2021;16(1):21. (10.1186/s13023-020-01627-x, [ Özet ]
22) Van der Werf A, Dekker IM, Meijerink MR, Wierdsma NJ, De van der Schueren MAE, Langius JAE. Skeletal muscle analyses: the agreement between non-contrast and contrast CT scan measurements of skeletal muscle area and mean muscle attenuation. Clin Physiol Funct Imaging. 2018;38(3):366-72. (10.1111/cpf.12422, [ Özet ]
23) Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg C, Thompson H, Walker P, Fu H, Dighe A, Griffin J-T, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon L, Nedjati-Gilani G, Riley S, Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly C-A, Ghani A-C, Ferguson N-M, Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669-77. (10.1016/S1473-3099(20)30243-7, [ Özet ]




