PROGNOSTIC IMPORTANCE OF HYPOXIA-INDUCIBLE FACTOR 1-ALPHA AND NUCLEAR FACTOR KAPPA B IN EARLY-STAGE GLOTTIC LARYNGEAL SQUAMOUS CELL CARCINOMA TREATED WITH RADIOTHERAPY
2Eskişehir Osmangazi Üniversitesi KBB Anabilim Dalı, KBB, Eskişehir, Turkey
3Eskişehir Osmangazi Üniversitesi Radyasyon Onkolojisi Anabilim Dalı, Radyasyon Onkolojisi, Eskişehir, Turkey
4Eskişehir Osmangazi Üniversitesi Patoloji Anabilim Dalı, Patoloji, Eskişehir, Turkey
Summary
Objective: Early-stage glottic laryngeal squamous cell carcinoma has good mortality and morbidity rates with radiotherapy. However, recurrent cases are at risk of increased morbidity and mortality. Therefore, the identification of new prognostic markers is needed.Material and Methods: Forty patients with early-stage glottic carcinoma managed with radiotherapy were included in this study. Immunohistochemical evaluation was performed for Nuclear factor-kappa B and Hypoxia-inducible factor 1-alpha from biopsy specimens. The relationship between overall survival and investigated parameters was analyzed by Kaplan-Meier and regression analysis.
Results: There were 11 recurrences and 20 deaths occurred during the follow-up period. There was no significant relationship between Nuclear factor-kappa B and Hypoxia-inducible factor 1-alpha staining and overall survival. There were no significant correlations between age, smoking status, differentiation, T stage, and anterior commissure involvement with Nuclear factor-kappa B and Hypoxia-inducible factor 1-alpha staining.
Conclusion: It seems pretreatment Nuclear factor-kappa B and Hypoxia-inducible factor 1-alpha expression levels do not correlate with overall survival in early-stage glottic carcinoma primarily managed with radiotherapy.
Introduction
Early-stage laryngeal carcinoma is defined as a T1-T2 tumor without evidence of any clinical local and/or distant metastases. Laryngeal carcinoma arises most commonly from the glottic region. The prognosis is better because of the possibility of early diagnosis and poor lymphatic drainage. The treatment goal for early-stage glottic carcinoma is a single-treatment modality, either open or endoscopic surgery or radiotherapy. Satisfaction with survival and local control rates can be achieved by using each treatment method. Although salvage laryngectomy can be performed in cases of radiotherapy failure, patients are usually at risk with increased complication rates. Therefore, identifying prognostic factors that can estimate radioresistance in the pretreatment period is crucial.Nuclear factor-kappa B (NF-κB) is a transcription factor normally located in the cytoplasm in an inactive form. NF-κB can be stimulated with hypoxia and chemical and physical stimulants. When activated, it translocates into the nucleus, where it activates genes related to angiogenesis, anti-apoptosis, and carcinogenesis [1]. Tumor hypoxia is a well-known factor that participates in treatment-resistant head and neck squamous cell carcinoma [2,3]. Hypoxia-inducible factor 1-alpha (HIF-1α) is another transcription factor that can be stimulated by hypoxia and other physical stimulants. HIF-1α also stimulates genes that participate in carcinogenesis. Therefore, both NF-κB and HIF-1α have been investigated in many tumors as prognostic factors[4]. Additionally, it has been shown that NF-κB may be responsible for radioresistance in laryngeal carcinoma[5,6].
In this study, we investigated the relationship between NF-κB and HIF-1α expression and overall survival in patients with early-stage glottic carcinoma managed primarily with radiotherapy.
Methods
Ethical approval for this study was obtained from the local ethics committee (approval number 2020/391) and was supported with the number 201111035 by Project Development and Support Unit of University. Patients diagnosed with early glottic laryngeal squamous cell carcinoma who underwent radiotherapy between November 2005 and January 2011 were included in the study. Patients with a history of surgery or radiotherapy in the head and neck region for any reason, and who were followed up for <1 year were excluded from the study. In total, 40 patients were eligible for inclusion in the study. Since our study is retrospective, informed consent was not obtained. The patients" clinical and pathologic parameters (e.g. age, smoking status, alcohol consumption, tumor differentiation, stage, anterior commissure involvement) and follow-up information (e.g. visit times, recurrence, or death) were recorded.The anterior commissure involvement was managed during direct laryngoscopy and with radiologic imaging. The patients were followed up according to the European Laryngeal Society guidelines[7].
Radiotherapy was given 5 days per week and 2 Gy daily per fraction. A total of 66-70 Gy (median 68 Gy) radiotherapy was applied. The patients were treated with a 64 MV photon and the field size was 6x6 cm. Elective neck radiotherapy was not given to any patient.
Surgical specimens were obtained for pathological examination. The tissues were sectioned at 4 mm thick. Routine deparaffinization and rehydration were applied. Immunohistochemical staining was performed using a Benchmark staining platform (Ventana Medical Systems Inc.) with a Ventana Universal DAB detection kit. HIF-1a Ab-4 (H1alpha 67) Mouse Mab and NF-κB p65 (Rel A) RB were used as primary antibodies. After staining, sections were dehydrated and covered with coverslips.
For each marker, at least 1000 cells were counted under a light microscope. Scoring was made without knowledge of clinical data.
HIF 1-a staining was divided according to the positive staining cell ratio into five groups; group 1: 0%, group 2: 1-10%, group 3: 11-50%, group 4: 51-80%, and group 5: 81-100%. For the statistical evaluation, groups 1, 2, and 3 were accepted as negative, and 4 and 5 as positive[8]. HIF 1-a staining of more than 50% of cells is seen in Figure 1.
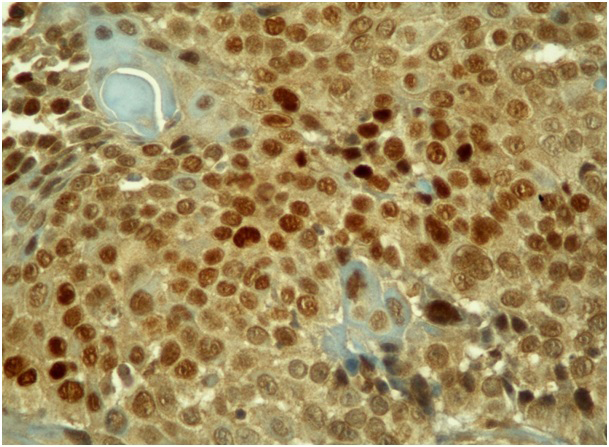 Büyütmek İçin Tıklayın |
Figure 1: HIF 1-a nuclear staining in more than 50% of tumor cells (H&E, x400). |
NF-κB staining was divided according to the nuclear and/or cytoplasmic staining cell ratio into three groups; group 1: <10% focal, group 2: 11-50% regional, and group 3: 51-100% diffuse. Also, three groups according to the staining intensity; group 1: weak, group 2: moderate, and group 3: strong. For the statistical evaluation, if one of the groups was 1, it was accepted as negative, if none of the groups were 1, it was accepted as positive[5]. The diffuse NF-κB staining is seen in Figure 2.
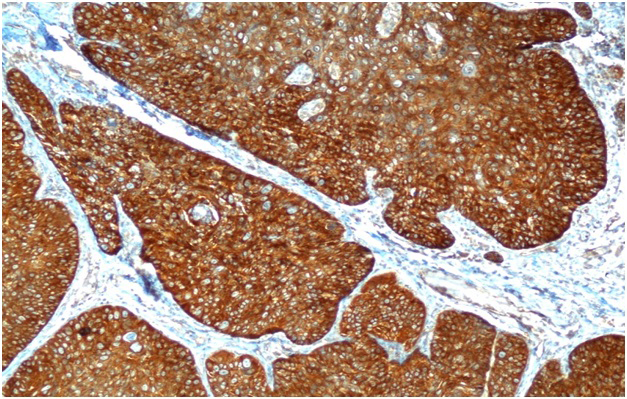 Büyütmek İçin Tıklayın |
Figure 2: Diffuse NF-κB cytoplasmic staining in tumor cells (H&E, x100). |
Statistical analysis
NCSS (Number Cruncher Statistical System, Kaysville, UT) 2007 software was used for the statistical analysis. Age, smoking, alcohol consumption, anterior commissure involvement, tumor stage, HIF-1a, and NF kappa B status considered for statistical evaluation. The relationship between HIF-1a and NF-κB status and age, smoking, alcohol consumption, anterior commissure involvement, and tumor stage was investigated using the Chi-square and Fisher's exact tests. The Brinkman Index [(number of cigarettes smoked per day) x (number of years smoked)] was used for the smoking status evaluation. Alcohol consumption was assessed as user and non-user. Logistic regression analysis was performed for univariate analysis. Overall survival [(OS); the time between the last date of radiotherapy to the date of the last follow-up or death from any cause) was calculated using the Kaplan-Meier analysis. In all analyzes, p<0.05 was considered to indicate statistical significance.
Results
The study population comprised 39 (97.5%) men and 1 (2.5%) woman with an average age of 64.70±9.22 years. The mean follow-up period, recurrence time, and time from radiotherapy to death were 60.85±31.19 months, 48.43±39.73 months, and 58.30±38.93 months, respectively. During the follow-up period, 11 (27.5%) recurrences and 20 (50%) deaths [9 (22.5%) of which were due to recurrence] occurred. Among 11 recurrent cases, three underwent total laryngectomy with neck dissection, one patient underwent neck dissection only, three patients received chemoradiotherapy, and four patients were out of follow-up after recurrences. The clinicopathologic data of the patients are presented in Table 1.Table 1: Clinicopathological characteristics of patients
Eighteen (45%) of the patients had NF-κB cytoplasmic staining, and 22 of the patients (55%) had both cytoplasmic and nuclear staining. According to the staining cell ratio; 2 (5%) patients were in the focal category, 17 (42.5%) were regional, and 21(52.5%) were in the diffuse category. According to the staining intensity, there were 12 (30%), 16 (40%), and 12 (30%) patients in groups 1, 2, and 3, respectively. Twelve (30%) patients were accepted as negative for NF-κB staining and 28 (70%) patients were accepted as positive.
For HIF-1α, according to the positive staining cell ratio, there were 5 (12.5%), 15 (37.5%), 7 (17.5%), 5 (12.5%), and 8 (20%) patients in groups 1, 2, 3, 4, and 5, respectively. Therefore, 27 (67.5%) patients were accepted as negative for HIF-1α staining and 13 (32.5%) patients were accepted as positive.
The relationship between clinicopathological parameters and HIF-1α and NF-κB expression status is presented in Table 2. There were no statistically significant relations (p>0.05).
Univariate analysis was performed for age (p=0.077), smoking status (p=0.960), alcohol consumption (p=0.791), tumor differentiation (p=0.990), anterior commissure involvement (p=0.613), NF-κB status (p=0.620), HIF-1α status (p=0.726), and stage (p=0.814). We did not perform multivariate analysis because only one parameter showed a p-value less than 0.1.
Discussion
In this study, we investigated the relationship between the expression of NF-κB and HIF-1α and OS in early-stage glottic carcinoma primarily managed with radiotherapy, with more than 5 years of follow-up. We also analyzed the effect of the T stage, the anterior commissure involvement, and tumor differentiation. We found no significant relationship between OS and NF-κB status, HIF-1α status, T stage, anterior commissure involvement, tumor differentiation, alcohol consumption, and smoking status. Also, there was no relationship between NF-κB and HIF-1α expressions with T stage, the anterior commissure involvement, tumor differentiation, alcohol consumption, and smoking status.It is well known that genetic and environmental factors play a role in tumorigenesis. Many cytokines, transcription factors, and markers are involved in different parts of this complicated process. These factors can be stimulated by various types of stimulants, such as hypoxia, bacterial and viral agents, radiation, chemical carcinogens, and chemotherapeutics[9]. The NF-κB family, composed of p50, p52, RelB, c-Rel, and p65/Rel, is usually located in the cytoplasm in an inactivated form[9-11]. When activated, NF-κB dimers translocate into the nucleus and stimulate more than a hundred genes responsible for inflammatory processes, metastasis, cellular motility, angiogenesis, and apoptosis[9-11]. It has been shown that NF-κB plays a role in the carcinogenesis of laryngeal carcinoma[6]. Additionally, NF-κB may be enhanced by chemotherapeutics and radiation, which causes chemo-radioresistance[9,10]. HIF-1α is a transcription factor that translocates to the nucleus and promotes the production of several genes under hypoxic conditions. Although hypoxia is a major stimulant for HIF-1α, it can also be stimulated by other factors[12]. Stimulation of HIF-1α causes induction of angiogenesis, cell proliferation, and apoptosis[4]. Also, it is known that NF-κB and HIF-1α have extensive crosstalks[4].
Schrijvers et al. investigated the prognostic ability of HIF-1α in patients with T1-T2 glottic carcinoma treated primarily with radiotherapy. There was no significant relation between HIF-1α and T and N status. They found that high levels of HIF-1α were significantly correlated with worse local control and OS[13]. By contrast, in another study, 271 patients with early glottic carcinoma treated primarily with radiotherapy were analyzed and no significant relation between HIF-1α levels and local control, cancer-specific survival, and the stage was observed[14]. Additionally, there was no significant relationship between HIF-1α status and advanced-stage larynx and hypopharynx carcinoma treated with an organ preservation protocol[15]. HIF-1α was also investigated in supraglottic T1-T2 tumors and no statistical significance was observed between HIF-1α levels and local control rates[12]. In another study, HIF-1α was investigated in surgically treated supraglottic larynx carcinoma. Although HIF-1α expression was correlated with T status, there was no significant correlation with survival[16]. Kyzas et al. showed that HIF-1α expression had no significant relationship with age, sex, histological grade, clinical stage, and survival[17]. A recent meta-analysis showed that HIF-1α had no predictive value in laryngeal carcinoma primarily managed with radiotherapy[18]. Our study results were similar to the literature. We observed no significant relationship between HIF-1α status and age, Brinkman Index, alcohol consumption, T stage, differentiation, and OS.
It has been shown that NF-κB plays role in laryngeal carcinogenesis, and in patients with head and neck carcinoma; NF-κB expression was significantly correlated with survival and local recurrence[6,11,19,20]. Yoshida et al. investigated NF-κB expression in early-stage laryngeal carcinoma primarily managed with radiotherapy. They reported that 77% of patients with radioresistance had positive NF- κB staining, which we have 70% in our study. They evaluated NF-κB expression in recurrent tumors and revealed that the positivity ratio was 90%. An increased expression ratio in recurrent tumors may indicate that NF-κB is one of the factors responsible for radioresistance. Also, they observed that positive NF-κB expression was correlated with poor local control and 5-year survival. In another study, Huang et al. investigated NF-κB expression in 78 patients with laryngeal carcinoma primarily managed with surgery[19]. They observed that NF-κB expression was significantly related to lymph node metastasis, T stage, and OS. Jiang et al.'s investigation included 89 patients with laryngeal carcinoma primarily managed with surgery, and they showed that NF-κB expression was significantly related to lymph node metastasis, T stage, clinical stage, and OS[21]. In our study, we observed no significant relationship between NF-κB expression and T stage, tumor differentiation, age, Brinkman Index, and anterior commissure involvement. Also, there was no relationship with OS.
It has been thought that anterior commissure involvement plays a crucial role in laryngeal carcinoma. Although some authors state that the anterior commissure area is resistant to cancer invasion and therefore plays a protective role, most authors agree that this area is prone to invasion, and invasion of the anterior commissure may ease the invasion of critically important areas such as the pre-epiglottic region and cricothyroid membrane. A recently published meta-analysis included only patients with T1 glottic carcinoma and showed that the anterior commissure invasion negatively affected 5-year local control rates, regardless of the treatment modality[22]. Also, in another meta-analysis, Eskiizmir et al. analyzed factors causing radiotherapy failure in early-stage glottic carcinoma and found the anterior commissure invasion to be among them[23]. However, another review showed that in early-stage laryngeal carcinoma, the anterior commissure invasion did not affect local control when evaluated as the anterior commissure (+/-)[24]. These conflicting results may be due to the difficulty in evaluating the anterior commissure invasion. In our study, we found no significant relationship between the anterior commissure invasion and local recurrence, and OS.
This study had several limitations. Since our study design is retrospective, it may be a patient affected by selection bias. Also, our study population is relatively small.
Reference
1) Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020 Sep 21;5(1):209. https://doi.org/10.1038/s41392-020-00312-6. [ Özet ]; PMCID: PMC7506548.
2) Kordbacheh F, Farah CS. Molecular Pathways and Druggable Targets in Head and Neck Squamous Cell Carcinoma. Cancers. 2021;13(14):3453. https://doi.org/10.3390/cancers13143453.
3) Alsahafi E, Begg K, Amelio I et al. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019 Jul 15;10(8):540. doi: 10.1038/s41419-019-1769-9. [ Özet ]; PMCID: PMC6629629.
4) D'Ignazio L, Rocha S. Hypoxia Induced NF-κB. Cells. 2016 ;5(1):10. https://doi.org/10.3390/cells5010010.
5) Yoshida K, Sasaki R, Nishimura H et al. Nuclear factor-kappaB expression as a novel marker of radioresistance in early-stage laryngeal cancer. Head Neck. 2010 ;32(5):646-655. https://doi.org/10.1002/hed.21239.
6) Monisha J, Roy NK, Bordoloi D et al. Nuclear Factor Kappa B: A Potential Target to Persecute Head and Neck Cancer. Curr Drug Targets. 2017;18(2):232-253. https://doi.org/ 10.2174/1389450117666160201112330. [ Özet ]
7) Simo R, Bradley P, Chevalier D et al. European Laryngological Society. ELS Recommendations for the follow-up of patients treated for laryngeal cancer. Eur Arch Otorhinolaryngol 2014 ;271(9):2469-79.
8) Kwon OJ, Park JJ, Ko GH et al. HIF-1α and CA-IX as predictors of locoregional control for determining the optimal treatment modality for early-stage laryngeal carcinoma. Head Neck. 2015 ;37(4):505-10. https://doi.org/10.1002/hed.23620. [ Özet ]
9) Wilczynski J, Duechler M, Czyz M. Targeting NF-κB and HIF-1 pathways for the treatment of cancer: part I. Arch Immunol Ther Exp (Warsz). 2011 ;59(4):289-299. https://doi.org/10.1007/s00005-011-0131-4.
10) Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010 ;1805(2):167-180. https://doi.org/10.1016/j.bbcan.2010.01.002.
11) Balermpas P, Michel Y, Wagenblast J et al. Nuclear NF-κB expression correlates with outcome among patients with head and neck squamous cell carcinoma treated with primary chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013 ;86(4):785-790. https://doi.org/10.1016/j.ijrobp.2013.04.001.
12) Wachters JE, Schrijvers ML, Slagter-Menkema L et al. Prognostic significance of HIF-1α , CA-IX, and OPN in T1-T2 laryngeal carcinoma treated with radiotherapy. Laryngoscope. 2013 ;123(9):2154-2160. https://doi.org/10.1002/lary.23831.
13) Schrijvers ML, van der Laan BF, de Bock GH et al. Overexpression of intrinsic hypoxia markers HIF1alpha and CA-IX predict for local recurrence in stage T1-T2 glottic laryngeal carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2008 ;72(1):161-169. https://doi.org/10.1016/j.ijrobp.2008.05.025.
14) Douglas CM, Bernstein JM, Ormston VE et al. Lack of prognostic effect of carbonic anhydrase-9, hypoxia inducible factor-1α and bcl-2 in 286 patients with early squamous cell carcinoma of the glottic larynx treated with radiotherapy. Clin Oncol (R Coll Radiol) 2013 ;25(1):59-65.
15) Bernstein JM, Andrews TD, Slevin NJ, West CM, Homer JJ. Prognostic value of hypoxia-associated markers in advanced larynx and hypopharynx squamous cell carcinoma. Laryngoscope. 2015 ;125(1):E8-E15. https://doi.org/10.1002/lary.24933.
16) Cabanillas R, Rodrigo JP, Secades P, Astudillo A, Nieto CS, Chiara MD. The relation between hypoxia-inducible factor (HIF)-1alpha expression with p53 expression and outcome in surgically treated supraglottic laryngeal cancer. J Surg Oncol. 2009 ;99(6):373-378. https://doi.org/10.1002/jso.21243.
17) Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Hypoxia-induced tumor angiogenic pathway in head and neck cancer: an in vivo study. Cancer Lett. 2005 ;225(2):297-304. https://doi.org/10.1016/j.canlet.2004.11.060.
18) Noordhuis MG, Kop EA, van der Vegt B et al. Biological tumor markers associated with local control after primary radiotherapy in laryngeal cancer: A systematic review. Clin Otolaryngol. 2020 ;45(4):486-494. https://doi.org/10.1111/coa.13540.
19) Huang C, Huang K, Wang C et al. Overexpression of mitogen-activated protein kinase kinase 4 and nuclear factor-kappaB in laryngeal squamous cell carcinoma: a potential indicator for poor prognosis. Oncol Rep. 2009 ;22(1):89-95.
20) Quintana A, Avilés FX, Terra X et al. Overexpression of the nuclear factor-kappa B (p65) in association with local failure in patients with head and neck carcinoma undergoing radiotherapy or chemoradiotherapy. Head Neck. 2013 ;35(3):370-375. https://doi.org/10.1002/hed.22979.
21) Jiang LZ, Wang P, Deng B et al. Overexpression of Forkhead Box M1 transcription factor and nuclear factor-κB in laryngeal squamous cell carcinoma: a potential indicator for poor prognosis. Hum Pathol. 2011;42(8):1185-1193. https://doi.org/ 10.1016/ j.humpath. 2010.06. 017.
22) Tulli M, Re M, Bondi S et al. The prognostic value of anterior commissure involvement in T1 glottic cancer: A systematic review and meta-analysis. Laryngoscope. 2020 ;130(8):1932-1940. https://doi.org/10.1002/lary.28395.




