THE ROLE OF LYMPHOCYTE-MONOCYTE RATIO IN DETERMINING PREDICTING RECURRENCE AND PROGNOSIS IN LARYNGEAL CANCER
2Manavgat State Hospital, KBB Kliniği, Antalya, Turkey
3Doğu Akdeniz Üniversitesi Tıp Fakültesi, KBB Kliniği, Lefkoşa, Kuzey Kıbrıs TC
4Özel Muayenehane, KBB Kliniği, İstanbul, Turkey
5SBÜ Şişli Hamidiye Etfal SUAM, Patoloji Anabilim Dalı, İstanbul, Turkey
Summary
Objective: Although inflammation plays a role in tumour rejection, it may also contribute to the progression of oncogenesis. Our goal was to investigate the lymphocyte-to-monocyte ratio (LMR) and its effects on cancer prognosis in laryngeal cancer patients.Methods: Patients treated in our clinic between 2003 and 2014 for a diagnosis of laryngeal squamous cell cancer were analysed retrospectively. A total of 164 patients were included in the study. Patients were divided into groups according to perivascular invasion status [PVI(+) and PVI(-)], thyroid cartilage invasion status [TCI(+) and TCI(-)], metastatic lymph node [LAP(+) and LAP(-)], perineural invasion status [PNI(+) and PNI(-)] and tumour stage (T1-T4). Differences of pretreatment LMR among the groups were evaluated. The relationships of LMR with survival time and recurrence were evaluated.
Results: There was a statistically significant inverse correlation between T stage (T1-T4a), and LMR level (p=0.001). LMR was significantly lower in the TCI(+) (p=0.004), LAP(+) (p=0.004), and PVI(+) (p=0.006) groups. The mean LMR in the recurrence (+) group was lower than in the recurrence (-) group for all stages, but a statistically significant difference was detected only in patients with early stage cancer (T1 and T2) (p=0.024 and 0.052, respectively). An increased recurrence rate was found in patients with an LMR≤3.01 (sensitivity:61.1%, specificity:60.9%). Among all of the prognostic factors, a low LMR was found to be the most important independent factor determining cancer-related death.
Conclusions: A decreased LMR, particularly in the early stages of laryngeal cancer, is associated with high recurrence rates and a poor prognosis.
Introduction
Head and neck cancer (HNC) is one of the six most common cancers. More than 90% of these tumours are squamous cell carcinomas (SCCs). Laryngeal cancer is the second most common of all head and neck malignancies after skin cancer, accounting for 2-5% of all malignancies and 25-30% of all HNCs [1]. The prognostic evaluation of patients with laryngeal cancer includes histological grade, clinical stage, tumour location and neck metastasis. Studies aiming to identify new prognostic factors have been performed [2]. Recent studies have investigated the relationship between inflammation and cancer prognosis, and between cell functions and the effects of chemokines in the context of the process of carcinogenesis.The presence of inflammatory cells, chemokines, and cytokines in the microenvironment during the development of tumoral tissue has been demonstrated in humans and experimental animal models [3]. Substantial evidence shows that although inflammation is a response to tumour rejection, it may also contribute to the progression of oncogenesis. This paradox can be explained by a dynamic switch from chronic smouldering inflammation, promoting cancer cell survival to florid, tissue-disruptive inflammatory reactions triggering cancer cell destruction [4,5].
The peripheral inflammatory blood cell response and pre-treatment inflammatory markers have been investigated as prognostic factors in many cancers [6-10]. The lymphocyte-to-monocyte ratio (LMR) has been studied in several cancers including lung, colorectal, pancreatic, ovarian, and HNC [11-14]. However, most studies on the LMR in HNC have assessed cancers of the nasopharynx or oral cavity, with few studies being concerned with other HNCs such as oropharyngeal, hypopharyngeal, and laryngeal cancers [14-20].
The purposes of this study were to determine the relationship between pre-treatment LMR in the peripheral blood and the prognosis of patients with laryngeal cancer, and to evaluate the relationship between LMR and clinicopathological characteristics such as thyroid cartilage invasion, cervical lymph node metastasis, and perineural and perivascular invasion.
Methods
We retrospectively screened the files of 207 patients diagnosed with laryngeal squamous cell cancer in our Otorhinolaryngology Clinic between 2003 and 2014, and who had a treatment plan implemented by the Otolaryngology and Oncology Departments of our hospital. Of these, 164 patients met the inclusion criteria for our study, which was approved by the Medical Ethics Board of xxx Hospital (Number 689).Patients with non-squamous cell carcinoma of the larynx, inflammatory diseases, such as chronic kidney disease, coronary heart disease, autoimmune disease, chronic liver disease, and active infection were excluded from the study. Patients without regular follow-up, or insufficient information in their files were also excluded. Informed consents were obtained from 134 patients, because 22 had died and no contact information was available for 8. Tumour region, T stage, metastatic lymph node status (LAP), perineural invasion status (PNI), perivascular invasion status (PVI), and thyroid cartilage invasion status (TCI) were investigated in each patient, radiologically and/or pathologically. Patients were staged according to the American Joint Committee on Cancer 2010 TNM staging system. Local and lymph node recurrences and metastases, and their development times during the follow-up period, were noted. Follow-up examination included fiberoptic laryngoscopy, neck ultrasonography, and magnetic resonance imaging. Recurrence was defined as any new mass found on imaging or laryngoscopic examination, histologically confirmed by biopsy or surgery. Patients were divided into groups based on their clinicopathological characteristics: PVI(+) or PVI(-), TCI(+) or TCI(-), LAP(+) or LAP(-), and PNI(+) or PNI(-).
White blood cell counts of lymphocytes and monocytes were obtained from blood samples taken before treatment. The LMR was calculated by dividing the absolute lymphocyte count by the absolute monocyte count. The type of treatment provided, including surgery, surgery plus radiotherapy (RT), RT, chemoradiotherapy (CRT), and surgery plus CRT, was determined, along with the surgical method (total or partial). The overall survival (OS) time was calculated for each patient. The relationships of clinicopathological characteristics and recurrence with OS were investigated.
Statistical Analyses
SPSS 15.0 for Windows software (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. For descriptive statistics, categorical variables are given as numbers and percentages, and for numerical variables data are provided as the mean ± standard deviation. Comparisons between two independent groups were made using Student"s t-test when the numerical variables were normally distributed, and with the Mann-Whitney U test if they were not. Independent numerical variables for more than two groups were compared by one-way ANOVA when there was a normal distribution, and with the Kruskal-Wallis test when there was not. Subgroup analyses were performed using the parametric Tukey test, and the nonparametric Mann-Whitney U test, with the Bonferroni correction applied. Relationships between numerical variables were examined by Spearman correlation analysis in the case of non-parametric data. Recurrence and survival rates of categorical variables were compared using Kaplan-Meier analysis. Factors associated with recurrence and death were examined by Cox regression analysis with the forward selection method. Statistical significance was accepted as p < 0.05.
Results
A total of 164 laryngeal cancer patients (158 males and 6 females) with an average age of 60.5 ± 9.8 years were included in the study. The mean follow-up time was 52.1 ± 33 months. Of the 164 patients, 55 were stage T1, 39 stage T2, 53 stage T3, 12 stage T4a, and 5 stage T4b. There were 63 glottic tumours, 12 glottic tumours with subglottic extension, 54 supraglottic tumours, and 32 transglottic tumours. In terms of pathological diagnosis, 34 patients had metastatic LAP, 33 had TCI, 27 had PVI, and 16 had PNI.The number of patients undergoing surgical treatment was 129 (78.6%), compared to 22 (13.4%) for RT only and 13 (7.9%) for CRT only. Of the 129 patients, 43 (26.2%) were treated with RT after surgery, and 2 (1.5%) received CRT after surgery. (Table 1)
There was a statistically significant inverse correlation between T stage (T1?4a) and LMR level (p = 0.001), except T4b. (Table 2) The mean LMR in the TCI (+) (2.7 ± 1.1) group was significantly lower than in the TCI(-) (3.5 ± 1.3) (p = 0.004) group. In the LAP (+) group, the mean LMR (2.7 ± 1.2) was significantly lower than in the LAP (-) group (3.5 ± 1.3) (p = 0.004). The mean LMR was lower in the PNI(+) group (2.8 ± 1.2) than the PNI(-) group (3.4 ± 1.4), but there was no statistically significant difference (p = 0.138). The mean LMR was significantly lower in the PVI (+) group (2.7 ± 1.3) than the PVI(-) group (35 ± 1.3) (p = 0.006). (Table 2) Recurrence occurred in 36 patients (21.9%), 16 (9.7%) patients died due to cancer, and 6 (3.6%) patients died from non-cancer diseases. (Table 1) The mean LMR in the recurrence (+) group was lower than in the recurrence (-) group for all stages. A statistically significant difference was detected only within the group of patients with early stage cancer (stages T1 and T2) (p = 0.024 and 0.052, respectively). In patients with early stage cancer (stages I and II) the mean LMR in the recurrence group was significantly lower than in the group without recurrence (p = 0.003). There was no significant difference between advanced-stage cancer patients with and without recurrence (stages III and IV). (Table 3) Using the forward selection method, a low LMR was found to be an important determinant of recurrence in early stage laryngeal cancer according to other prognostic factors. (Table 4) The cut-off LMR value for recurrence was 3.01; i.e. recurrence was particularly high in patients with an LMR ? 3.01 (sensitivity: 61.1%, specificity: 60.9%). (Table 5 and Figure 1) There was no statistically significant relationship between survival time and LMR. (Table 6) In the Cox regression model created to examine factors associated with cancer related death like cartilage invasion, LAP, perivascular and perineural invasion, stage, the LMR, and tumour region, a low LMR was found to be the independent most important factor by using the forward selection method. (Table 7)
Table 2: LMR distribution according to pathological diagnostic features and T status
Table 4: İmportance of LMR among the factors determining reccurence according to T stage.
Table 6: Relation between LMR and survival times.
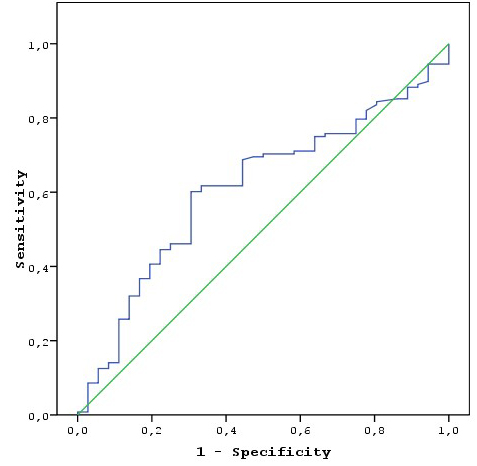 Büyütmek İçin Tıklayın |
Figure 1: Recurrence by LMR: sensitivity-specificity relationship (receiver operating characteristic curve analysis). |
Table 7: Association of LMR with cancer related death as an independent factor.
Discussion
Various inflammatory biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), red cell distribution width, and C-reactive protein have been investigated in the context of cancer prognosis [5-8]. The LMR is another inflammatory biomarker. However, the exact mechanism behind the relationship of LMR with cancer prognosis remains poorly understood. It is thought that lymphocytes have cytotoxic effects on tumoral cells, whereas neutrophils, platelets, and macrophages show anti-tumour effects, and also stimulate tumour cell proliferation and angiogenesis by secreting chemoattractant agents and various angiogenic factors. Therefore, the balance between different types of inflammatory cells may affect tumour prognosis [3,4,2,22,23]. An elevated LMR implies an increase in lymphocytes relative to monocytes. Higher amounts of infiltrating lymphocytes correlate with improved prognosis [24]. However, increased levels of monocyte-derived macrophages are associated with increased tumour aggressiveness and poorer survival outcomes [25]. It is assumed that tumour microenvironment mediators, such as TNF-α, vascular endothelial growth factor, and epidermal growth factor may be responsible for these results. [25,26]. The prognostic ability of LMR may be related to its role in the pro- versus anti-tumour activity balance in the immune system. A review of the literature using the Web of Science, Pubmed, Google Scholar, and Scopus databases revealed several studies on LMR and the prognosis of many cancers, including pancreatic, ovarian, colorectal, breast, gastric, and lung cancers [11,12,13,27,28,29] but only nine studies and one meta-analysis on LMR and laryngeal cancer prognosis [14,30-38]. (Table 8) Among the nine studies, seven had results specific to only laryngeal cancer prognosis and its relationship to LMR; the other two evaluated laryngeal cancer prognosis together with other HNCs. The meta-analysis included all HNCs: nasopharyngeal, oral cavity, laryngeal, oropharyngeal, and hypopharyngeal cancers [14]. We found two other studies that investigated the relationships of LMR with benign, precancerous, and malignant laryngeal lesions [39,40]. The behaviour and prognosis of HNC can vary according to the area affected. In our opinion, HNC must be examined separately according to location. Some studies indicate that the content of the tumour microenvironment varies greatly among different types of laryngeal tumours, i.e. supraglottic, glottic and subglottic tumours. Results from the in vitro studies of Goswami et al. demonstrated that the extent of expression of M2 phenotypic features in macrophages reached a maximum after exposure to supraglottic laryngeal tumour cell lysates rather than glottic or subglottic lysates. Co-culture of such M2 macrophages with T cells from healthy donors resulted in a larger decrease in the activation of T cells and T cell-mediated tumour cell cytotoxicity relative to glottic and subglottic lysates [41]. We investigated only the laryngeal cancer-LMR relationship, without subgrouping according to laryngeal subtypes.Most studies were based on preoperative LMR status, although some considered both pre- and postoperative LMR [30-36,38]. In one study, delta-LMR was used, calculated as the difference between LMR measured during treatment and the baseline LMR value [37]. In our study, we only investigated preoperative LMR status. Among the nine studies, six treated patients with primary surgery, and three with CRT/RT [30-38]. We included all treatment types, i.e. primary surgery, and primary RT and CRT.
Clinicopathological characteristics and relationship to LMR
We investigated not only the effect of LMR on laryngeal cancer prognosis, but also the relationship between clinicopathological characteristics and LMR. Our results were similar to the literature in some respects. Changes in inflammatory balance may be a consequence of these clinicopathological characteristics, or the inflammatory balance may be an independent prognostic factor promoting the formation of these clinicopathological characteristics. Most literature studies considered the T stage and lymph node status as the clinicopathological characteristics of interest. In most of the research, a lower LMR was related to a higher T stage, clinical stage and presence of metastatic lymph nodes, but some studies showed no relationship of the LMR with these clinicopathological characteristics. We investigated T status, TCI, lymph node status, PNI, and PVI. We found that increased T stage was related to decreased LMR, except the T4b stage, likely because we had very few patients at that stage (n = 5). (Table 2) LMR was lower in the presence of metastatic lymph node invasion, TCI, PVI, and PNI (Table 3). Particularly in the TCI(+), LAP(+), and PVI(+) groups, a statistically significant difference was observed. Results of previous studies on the LMR relationship to prognostic factors are documented in the Supplemental Table 8.
Table 8: Published studies about LMR and Laryngeal cancer prognosis
Kano et al. reported that LMR was the inflammatory marker most strongly related to laryngeal cancer according to the PLR and NLR. A high preoperative LMR correlated with lower stage laryngeal cancer (T1-T2, and clinical stage I-II). They did not find a statistically significant correlation between N status and LMR [30]. Hsueh et al. reported that preoperative lower LMR correlated with larger tumour size, higher tumour stage, and the presence of metastatic lymph nodes. They did not find any relationship between smoking, alcohol consumption, or differentiation grade with LMR [31]. Chen L. et al. reported no statistically significant associations of preoperative LMR with other prognostic factors, such as N status, T stage, clinical stage, tumour location, and differentiation [32]. Zhou et al. investigated the preoperative albumin/globulin ratio (AGR), together with NLR, PLR, and LMR, as a prognostic factor in patients with only laryngeal cancer treated by primary surgery. They observed that higher T stage, higher clinical stage, and positive lymph node status were significantly correlated with a lower LMR. They reported that AGR was a better prognostic factor than NLR, PLR, and LMR in laryngeal SCC patients [34]. Xun et al. observed no statistically significant associations of T stage, N stage, differentiation grade, or clinical stage with LMR, but reported that high NLR and PLR were significantly related to higher T and N stages [35]. Chuang et al. investigated the LMR and Systemic Inflammation Response Index as prognostic factors among patients with only laryngeal cancer, treated by intensity-modulated radiation therapy. They reported that high T stage and advanced clinical stage were significantly related to a low LMR. They compared N0, 1, 2, and 3a with N3b, and did not find any statistically significant difference in terms of the associations with the LMR [36]. Tham et al. investigated preoperative NLR, PLR, and the LMR as prognostic factors in oral cavity, oropharyngeal and laryngeal cancer patients treated by primary surgery. In terms of prognostic factors, tumour differentiation, T stage, N stage, Eastern Cooperative Oncology Group (ECOG) score, Karnofsky Performance Status, Adult Comorbidity Evaluation-27 score, surgical margins, and HPV infection, and their relationships to LMR, were investigated. They reported that positive metastatic lymph nodes and higher ECOG status were significantly related to a lower LMR. Significantly more of comorbidities were seen with a lower LMR, and HPV-positive patients had a significantly higher LMR than HPV-negative patients [33].
Relationship of LMR to prognosis
In terms of the recurrence rate, we found that the LMR was significantly lower in the early stage (T1 and T2) recurrence (+) laryngeal cancer group. In the later stages, there was no significant difference between the recurrence and non-recurrence groups. Esspecialy in early stage cancers low LMR is found most important factor that determining reccurence according to other prognostic factors. Other research did not evaluate the relationship between recurrence and the LMR according to cancer stage (early vs. advanced). Recurrence was higher, particularly in patients with an LMR ? 3.01 (sensitivity: 61.1%, specificity: 60.9%). We suspect that in the earlier stages, the inflammatory balance is more important. In the later stages, the volume of the tumour is larger and invasion is greater; because of this, investigation of inflammatory balance could be more complicated. In our research, the rate of organ-preserving treatment (total laryngectomy, 38.4%) was lower among advanced-stage (stages III and IV) patients. This may be another cause of the statistical insignificance in the advanced-stage cancer groups in terms of recurrence. No study in the literature investigated the relationship between recurrence rate and the LMR according to stage. We did not find a statistically significant relationship between cancer-specific OS and LMR, but we did find that the factor most strongly associated with mortality was a low LMR. Published studies on the LMR in laryngeal cancer patients, in terms of OS and disease-free survival (DFS) times, are summarised in Supplemental Table 8. A lower preoperative LMR was associated with significantly worse OS and DFS. Chen L. et al. reported that a high postoperative, but not preoperative, LMR was related to a poor prognostic outcome [32]. Zhou et al. reported that lower LMR was related to a low OS, but not DFS [34]. Chen H. et al. did not find any relationship between LMR and OS or DFS [38]. Lin CH reported that delta-LMR was an independent prognostic factor for both metastasis-free survival and OS [37].
Of note, the LMR cut-off value varied among studies. The maximum value was 3.22, and the minimum was 2.01 [30-38]. LMR can be an independent prognostic factor for laryngeal cancer but it also shows a close relationship with other prognostic factors.
Limitations
Limitations of our study included the use of different treatment modalities and differences in the distribution thereof.
Conclusion
Determination of the LMR through analysis of peripheral blood is simple and inexpensive. The LMR is an important prognostic factor for laryngeal cancer, related to thyroid cartilage invasion, lymph node metastasis, advanced T stage, and perivascular invasion. A decreased LMR significantly predicted the risk of recurrence, particularly in early stage laryngeal cancer patients.Conflicts of Interest and Source of Funding: None
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/zc35V1
Reference
1) Ciolofan MS, Vl?escu AN, Mogoant? CA, et al Clinical, histological and immunohistochemical evaluation of larynx cancer. Curr Health Sci J. 2017;43:367-75. [ Özet ]
2) Vielba R, Bilbao J, Ispizua A, et al. p53 and cyclin D1 as prognostic factors in squamous cell carcinoma of the larynx. Laryngoscope. 2003 Jan;113:167-7. [ Özet ]
3) Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflamation. Nature.2008 24;454:436-44. [ Özet ]
4) Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008 Mar 1;371:771-83. [ Özet ]
5) Fang HY, Huang XY, Chien HT, et al. Refining the role of preoperative C-reactive protein by neutrophil/lymphocyte ratio in oral cavity squamous cell carcinoma.Laryngoscope. 2013 Nov;123:2690-9. [ Özet ]
6) Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008 Jan;34:55-60. [ Özet ]
7) Young CA, Murray LJ, Karakaya E, Thygesen HH, Sen M, Prestwich RJ. The Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Oropharyngeal Carcinoma Treated with Chemoradiotherapy. Clin Med Insights Oncol. 2014 Jun 29;8:81-6. [ Özet ]
8) Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck. 2015 Jan;37:103-10. [ Özet ]
9) Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009; 197:466-72. [ Özet ]
10) Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009; 58:15-23. [ Özet ]
11) Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg. 2018 Jul;55:128-138. [ Özet ]
12) Gong J, Jiang H, Shu C, et al. Prognostic value of lymphocyte-to-monocyte ratio in ovarian cancer: a meta-analysis. J Ovarian Res. 2019 May 31;12:51. [ Özet ]
13) Wang Y, Huang D, Xu WY, et al. Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Non-Small Cell Lung Cancer: A Meta-Analysis. Oncol Res Treat. 2019;42:523-531. [ Özet ]
14) Tham T, Olson C, Khaymovich J, Herman SW, Costantino PD. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2018 Jul;275:1663-1670. [ Özet ]
15) Li J, Jiang R, Liu WS, et al. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One. 2013 Dec 27;8:e83069. [ Özet ]
16) Lu A, Li H, Zheng Y, et al. Prognostic Significance of Neutrophil to Lymphocyte Ratio, Lymphocyte to Monocyte Ratio, and Platelet to Lymphocyte Ratio in Patients with Nasopharyngeal Carcinoma. Biomed Res Int. 2017;2017:3047802. [ Özet ]
17) Jiang R, Cai XY, Yang ZH, et al. Elevated peripheral blood lymphocyte-to-monocyte ratio predicts a favorable prognosis in the patients with metastatic nasopharyngeal carcinoma. Chin J Cancer. 2015 Jun 10;34:237-46. [ Özet ]
18) Ong HS, Gokavarapu S, Wang LZ, et al. Low pretreatment lymphocyte?monocyte ratio and high platelet?lymphocyte ratio indicate poor cancer outcome in early tongue cancer. J Oral Maxillofac Surg. 2017;75:1762-74 [ Özet ]
19) Lin GN, Peng JW, Liu DY, Xiao JJ, Chen YQ, Chen XQ. Increased lymphocyte to monocyte ratio is associated with better prognosis in patients with newly diagnosed metastatic nasopharyngeal carcinoma receiving chemotherapy. Tumor Biol. 2014;35:10849-54. [ Özet ]
20) Li XH, Chang H, Xu BQ et al. An inflammatory biomarkerbased nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med. 2016;6:310-19. [ Özet ]
21) Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001; 357:539-545. [ Özet ]
22) Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011 Feb;9:237-49. [ Özet ]
23) Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res 2006;4:221-33. [ Özet ]
24) Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011; 105:93-103. [ Özet ]
25) Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2006;4:71-8. [ Özet ]
26) Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153:587. [ Özet ]
27) Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis. Clin Chim Acta. 2018 Jun;481:142-46. [ Özet ]
28) Hu RJ, Liu Q, Ma JY, Zhou J, Liu G. Preoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: A meta-analysis. Clin Chim Acta. 2018 Sep;484:1-6. [ Özet ]
29) Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: A meta-analysis. Int J Surg. 2018 Feb;50:67-71. [ Özet ]
30) Kano S, Homma A, Hatakeyama H, et al. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck. 2017 Feb;39:247-253. [ Özet ]
31) Hsueh C, Tao L, Zhang M, et al. The prognostic value of preoperative neutrophils, platelets, lymphocytes, monocytes and calculated ratios in patients with laryngeal squamous cell cancer. Oncotarget. 2017 Mar 15;8:60514-60527. [ Özet ]
32) Chen L, Zeng H, Yang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer. 2018 Aug 13;18:816. [ Özet ]
33) Tham T, Olson C, Khaymovich J, Herman SW, Costantino PD. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2018 Jul;275:1663-70. [ Özet ]
34) Zhou T, Yu ST, Chen WZ, Xie R, Yu JC Pretreatment albumin globulin ratio has a superior prognostic value in laryngeal squamous cell carcinoma patients: a comparison study. J Cancer. 2019 Jan 1;10:594-601. [ Özet ]
35) Xun Y, Wang M, Sun H Shi S, Guan B, Yu C Prognostic Analysis of Preoperative Inflammatory Biomarkers in Patients With Laryngeal Squamous Cell Carcinoma. Ear Nose Throat J. 2020 Jul;99:371-78. [ Özet ]
36) Chuang HC, Tsai MH, Lin YT, et al. The Clinical Impacts of Pretreatment Peripheral Blood Ratio on Lymphocytes, Monocytes, and Neutrophils Among Patients with Laryngeal/Hypopharyngeal Cancer Treated by Chemoradiation/Radiation. Cancer Manag Res. 2020 Sep 25;12:9013-21. [ Özet ]
37) Lin CH, Chou WC, Wu YY, et al. Prognostic significance of dynamic changes in lymphocyte-to-monocyte ratio in patients with head and neck cancer treated with radiotherapy: results from a large cohort study. Radiother Oncol. 2021 Jan;154:76-86. [ Özet ]
38) Chen H, Song S, Zhang L, Dong W, Chen X, Zhou H. Preoperative platelet-lymphocyte ratio predicts recurrence of laryngeal squamous cell carcinoma. Future Oncol. 2020 Feb;16:209-217. [ Özet ]
39) Kara A, Guven M, Demir D, Yilmaz MS, Gundogan ME, Genc S. Are calculated ratios and red blood cell and platelet distribution width really important for the laryngeal cancer and precancerous larynx lesions. Niger J Clin Pract. 2019 May;22:701-706. [ Özet ]
40) Wu DQ, Huang XS. The significance of lymphocyte to monocyte ratio in peripheral blood of patients with benign and malignant laryngeal lesions. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017 Jun 5;31:835-38. [ Özet ]
41) Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, Baral R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017 Jun;316:1-10. [ Özet ]




