THE IMPACT OF CRANIAL MRI NOISE ON INNER EAR
2Sultan 2. Abdülhamid Han Eğitim ve Araştırma Hastanesi, Nöroloji, İstanbul, Turkey
3Sultan 2. Abdülhamid Han Eğitim ve Araştırma Hastanesi, Biyokimya, İstanbul, Turkey
4Sultan 2. Abdülhamid Han Eğitim ve Araştırma Hastanesi, Radyoloji, İstanbul, Turkey
Summary
Objective: Exposure to intense noise may result in damage to the inner ear hair cells. Therefore, high-level noise generated by magnetic resonance imaging (MRI) causes concern for potential damage to the inner ear. This study aimed to evaluate any damage to the inner ear in patients undergoing cranial MRI without any ear plugs.Material and Methods: Thirty four patients who underwent cranial MRI without any ear plugs were reviewed prospectively. Audiometry , distortion product otoacoustic emission (DPOAE) level and prestin-level were evaluated respectively; a) two hours before MRI, b) twenty-four hours after MRI, and c) fourteen days after MRI.
Results: No statistical difference was found between three audiometric assessments in both ears for each frequency. There was no statistical difference between the three DPOAE assessments in both ears for each frequency. Although the level of prestin increased slightly in the second assessment, there was no statistical difference between the three prestin level assessments (P=0.31).
Conclusion: Although the level of prestin slightly increases, cranial MRI noise does not cause hearing loss in any patient.
Introduction
Inner ear hair cells are sensitive receptors that are capable of detecting mechanical sound and help to unravel the sound to understand the spoken language. They can be damaged in case of intense noise. Especially the outer hair cells are the first and most affected cells in the cochlea. In case of acoustic trauma, hair cells lose their structural integrity and start the self-disruption process due to oxidative stress. Finally, it leads to complete hair cell death by activation of different cellular death pathways [1,2].Otoacoustic emissions (OAEs) are the sounds of the cochlea originating from the sensory hair cell. Although OAEs can be recorded silently, they are more commonly measured in response to acoustic stimulation. Continuous sinusoidal stimuli evoke distortion product otoacoustic emissions (DPOAEs) that can be used to evaluate cochlear outer hair cells [3]. DPOAE is a type of OAE examination which can detect mild hearing loss at high frequencies. DPOAE test fails in case of irregularity, decreased number or loss of outer hair cells (OHC) [4].
Audiometry is performed routinely at conventional frequencies [between 0.25 and 8 kilohertz (kHz)] for the measurement of hearing threshold shift. However, researches indicate that hearing thresholds in extended high frequency (8-20 kHz) might be affected by noise earlier [5,6], which means that extended high-frequency audiometry may identify individuals with beginning hearing loss not yet remarkable in conventional audiometry [7].
Prestin, a motor membrane protein expressed in the outer hair cells (OHCs) of the cochlea; detects membrane potential and directs rapid length changes in OHCs. If OHC enters the apoptosis path, supporting cells phagocytosis also begins [8,9]. Many cellular contents, including structural proteins, are released into the circulation. For all these reasons, prestin is a unique marker to be used as a biomarker of the inner ear function and possible hearing loss [10].
A serious acoustic noise occurs during magnetic resonance imaging (MRI). This sound is a result of lorentz forces acting on gradient coils. As the gradient current changes direction, the gradient windings vibrate in their assemblies and sound waves propagate [11]. As the amount of noise reported increases significantly after the first measurements published in 1989, there is an increased safety concern on MRI [12]. Sound pressure levels (SPL) were between 82 and 93 dB in this study, which was conducted on a 0.35 Tesla (T) system at that time. However, recent studies have proved the presence of much higher noise levels that can cause hearing loss in 1.5T and 3T systems [13,14].
In this study, we aimed to evaluate the damage to the inner ear with high frequency audiometry, DPOAE and prestin levels in patients undergoing cranial MRI without ear plugs.
Methods
ParticipantsIn our cross-sectional study, data from 34 adult patients who underwent cranial magnetic resonance imaging (MRI) between July 2019 and October 2019 were reviewed prospectively. Ethics approval was obtained from the local Ethical Committee (2019/75), and an informed consent was obtained from all patients. The mean age of the 18 female and 16 male patients was 39.23+12.20 years (20-62 years).
All patients included in the study were referred to our neurology outpatient clinic for headache, and after the neurological examination and clinical evaluation, MRI was indicated for elimination of secondary headaches.
Patients were excluded if they met any of the following conditions: 1) older than 65 years old; 2) ear disease (e.g., external auditory canal occlusion, otitis media, trauma, perforation of tympanic membrane, ear tumors); 3) exposure to ototoxic drugs; 4) prolonged exposure to noise; 5) any systemic diseases which may affect auditory pathways (e.g., diabetes mellitus, hypertension, kidney disease, thyroid disease and autoimmune diseases); and 6) a history of infectious diseases which may affect hearing (e.g., meningitis, mumps, measles and syphilis).
Hearing Assessments
Hearing assessments of the patients were done as follows; a) first assessment: two hours before MRI, b) second assessment: twenty-four hours after MRI, and c) third assessment: fourteen days after MRI. Hearing levels [250-16000 Hertz (Hz)] were measured in a soundproof cabin using the Interacoustics AC-40 (Interacoustics A / S, Denmark) clinical audiometer. DPOAE measurements were made in an acoustically isolated room with a Madsen Capella system (GN Otometrics, Denmark). Two primary pure tones were used at frequencies F1 and f2, where f1 was set to 65 dB SPL and f2 to 55 dB SPL (f2 / f1 ratio = 1.22). DPOAE amplitudes were recorded as a function of the frequency f2 at 500?8,000 Hz. Acceptance criterion for DOPAE response is determined to be minimum 0 dB SPL level and signal-to-noise ratio ?6 dB SPL at each frequency f2 [15].
Enzyme-linked Immunosorbent Assay (ELISA)
Blood samples were taken from the patients before all hearing assessments (three times). Following centrifugation (2.000 rpm) for 10 minutes, the resulting supernatant (plasma) was collected and stored at-80° Celcius. Prestin concentration was measured using Human Prestin (SLC26A5) Elisa Kit (YLbiont human prestin elisa kit, Shunghai YL Biotech Co., Ltd) as described in the manufacturer"s instruction manual by a blinded biochemist. The optical density in the wells of the Elisa microplate was measured at 450nm using a Biotek ELx808 plate reader and data were compiled using the KCJunior software package (BIO-TEK instruments, Inc., Winooski, VT, U.S.A.).
Noise Exposure
All patients" cranial MRI were performed on a 1.5T MRI scanner (Magnetom Symphony, Siemens Medical Systems, Erlangen, Germany). During a 9 minutes and 57 seconds procedure, the same standard conditions applied to all patients in the radiology department, and patients completed MRI scans without any noise canceling ear plugs. Duration of each different 7 sequences on cranial MRI and minimum and maximum noise levels in each sequence were recorded. Noise levels were measured by an engineer using a sound level meter (Extech407740, Taichung, TAIWAN), that was mounted 30 centimeter (cm) from the head coil.
Power analysis
Power analysis was performed in these groups using PS-Power and Sample Size Calculation software as in the study of Liba et al. during which they examined the concentrations of prestin after inducing ototoxicity with cisplatin in rats and pigs [16]. As a result of the analysis, it was calculated that at least 2 subjects were required for prestin testing. However, since parametric tests with a sample size of at least 30 offer more powerful statistical approaches, at least 30 subjects were planned for this study.
Statistical Analysis
SPSS 21.0 (SPSS, Inc., Chicago, IL, USA) was used to perform all statistical analyses. The descriptive statistics were expressed as the mean ± standard deviation (SD). Repeated Measures Analysis of Variance analysis was used for prestin in groups.
The data for hearing changes in each frequency were analyzed using repeated measure ANOVA. Harmony of covariance was measured by Mauchly's test of Sphericity, whereas f test was used in harmonized covariance in Sphericity assumed conditions. For all analyses, a p value <0.05 was considered statistically significant.
Results
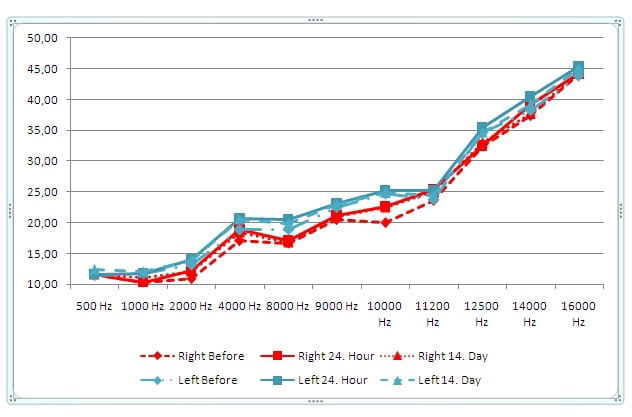
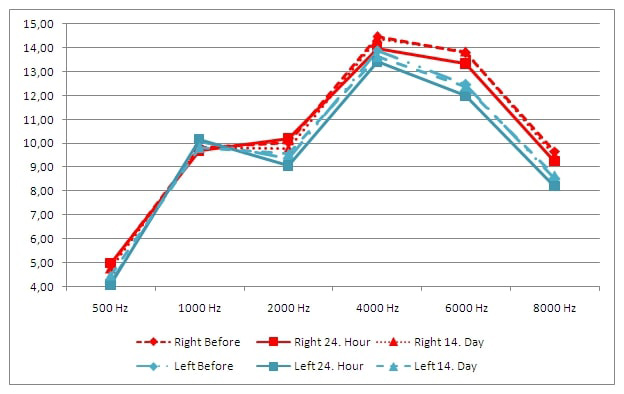
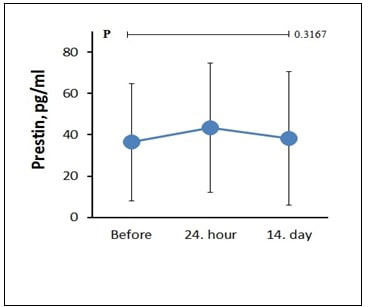
Duration of each different 7 sequences on cranial MRI and minimum and maximum noise levels in each sequence are shown in Table 1. The difference in the average hearing level for each frequency did not exceed 5 dB between the three audiometric assessments in both ears. Also, no statistical difference was found between the three audiometric assessments in both ears for each frequency (500, 1000, 2000, 4000, 8000, 9000, 10000, 11200, 12500, 14000 and 16000 Hz); (Right ear P=0.999, P=0.463, P=0.349, P=0.461, P=0.921, P=0.821, P=0.251, P=0.470, P=0.510, P=0.635, P=0.129, respectively), (Left ear P=0.602, P=0.857, P=0.672, P=0.309, P=0.631, P=0.873, P=0.882, P=0.562, P=0.885, P=0.330, P=0.437, respectively) (Figure 1). The difference in the average change for each frequency did not exceed 0.5 between the three DPOAE assessments in both ears. No statistical difference was found between the three DPOAE assessments in both ears for each frequency (500, 1000, 2000, 4000, 6000 and 8000 Hz); (Right ear P=0.901, P=0.982, P=0.759, P=0.689, P=0.440, P=0.530, respectively) and (Left ear P=0.967, P=0.310, P=0.906, P=0.523, P=0.363, P=0.264, respectively) (Figure 2). Mean of prestin levels were measured as 36.39+28.45, 43.39+31.44, 38.24+32.47, on the first, second and third assessments, respectively (Figure 3). No statistical difference was found between the three prestin level assessments (P=0.31).Table 1: Duration of time and noise level in cranial MRI.
 Büyütmek İçin Tıklayın |
Figure 1: Frequency audiometry results on the first, second and third assessments. |
 Büyütmek İçin Tıklayın |
Figure 2: DPOAE results on the first, second and third assessments. |
 Büyütmek İçin Tıklayın |
Figure 3: Prestin levels on the first, second and third assessments. |
Discussion
MRI is one of the most widely used imaging studies today. It is used routinely in clinical and basic research studies, as it allows mapping of the internal structure of the body. Its non-invasiveness and non-radiation emission are the most positive aspects. Unfortunately, it has a disadvantage of producing high acoustic noise which can cause stereocilia damage. In more serious cases, it can cause mechanical trauma to OHCs and Corti's organ [17]. Revadi et al. presented a patient with sudden hearing loss after 3T lumbosacral MRI, who was exposed to 118.4 dB [18]. Hattori et al. found that the noise ranged from 125.7-130.7 dB for 3T devices and 101.8-111.7 dB for 1.5T devices [19].Wagner et al. indicated that the noise was in a range of 79.5-86.5 dB for 1.5 T [20]. In our study, all sequences of cranial 1.5T MRI were in a range of 71.3-102.9 dB.In conventional audiometry, which is performed routinely, 3000, 4000 and 6000 Hz are the frequencies most affected by noise [21,22]. Higher hearing thresholds are also important when exposed to noise. This can be explained by cochlear anatomy and vascularization of the cochlear base [23]. Some studies have shown that frequency thresholds higher than 8 kHz are more sensitive to noise than conventional thresholds (ie 8 kHz and lower) [24,25,26]. In our study, the difference in the average hearing level for each frequency did not exceed 5 dB between the three audiometric assessments in both ears.
Using DPOAE tests to monitor noise-related damage in OHCs is a common strategy. They have demonstrated high sensitivity to noise damage, not only by well-known DPOAEs to identify OHC damage due to cisplatin or aminoglycoside treatments, but also by deficiencies in the DPOAE amplitude reported to be related to noise [27,28]. In our study, the difference in the average change for each frequency did not exceed 0.5 between the three DPOAE assessments in both ears. However, in the second DPOAE assessment of both ears, the highest decrease was found in the values of 4000, 6000 and 8000 Hz among all frequencies compared to the first DPOAE assessment. The differences between the third and the first DPOAE assessments at 4000, 6000 and 8000 Hz were reduced.
Prestin is localized in the lateral plasma membrane of OHCs. The electromotility here is thought to be the physical process underlying the cochlear amplifier. For this reason, prestin plays a central role in cochlear sensitivity and adjustment [29,30]. If the OHCs enter the apoptosis pathway, the supporting cells become phagocyted and cellular contents, including structural proteins, circulate freely. Therefore, prestin is an ideal marker of inner ear damage and possible hearing loss [9]. We found that prestin level was slightly increased in the second assessment, which shows that the outer hair cells of the cochlea were slightly damaged.
In the study of Brummett et al., 43% of patients without hearing protection devices suffered from transient mild hearing loss compared to 10% of the control group with hearing protection devices after 40 minutes of noise exposure in a 0.65T MRI device [31]. Radomskij et al. compared hearing loss between two groups of patients with or without ear plugs. They found greater changes in OAEs among those without ear plugs, which remained in 68% of participants up to 10 minutes after the after MRI procedure [32]. In our study, although no patient used ear plugs in cranial MRI during a period of 9 minutes 57seconds, there was no sudden hearing loss in any of the patients. We believe that the reason for the absence of any sudden hearing loss is the short duration of cranial MRI.
Conclusion
Patients are exposed to noise, although it is for a short time, during cranial MRI. No sudden hearing loss was detected in any patients, but there was a slight increase in the level of prestin. Therefore, we recommend the use of ear plugs even during short MR imaging.Acknowledgements: The authors thank the supporter.
Funding: This work was supported. (Grant No. 2018/056).
Competing interest: The authors have declared that they have no conficts of interest.
Reference
1) Waqas M, Gao S, Salam I, Ali MK, Ma Y, Li W. Inner Ear Hair Cell Protection in Mammals against the Noise-Induced Cochlear Damage Neural Plast. 2018;2018:3170801. [ Özet ]
2) Hill K, Yuan H, Wang X, Sha SH. Noise-InducedLoss of Hair Cells and Cochlear Synaptopathy Are Mediated by the Activation of AMPK. J Neurosci. 201613;36:7497-510. [ Özet ]
3) Lin X, Shan X, Lin S, Shu B, Wang Y, XiaoW. Is Sensorineural Hearing Loss Related to Chronic Rhinosinusitis Caused by Outer Hair Cell Injury? MedSciMonit. 2019;25:627-36. [ Özet ]
4) Kemp DT. Otoacousticemissions, their origin in cochlear function, and use. BrMedBull, 2002;63:223-41. [ Özet ]
5) Porto MA, Gahyva DL, Lauris JR, Lopes AC. Audiometric evaluation in extended high frequencies of individuals exposed to occupational noise. Pro-fono. 2004;16:237-50. [ Özet ]
6) Wang Y, Yang B, Li Y, Hou L, Hu Y, Han Y. Application of extended high frequency audiometry in the early diagnosis of noise-induced hearing loss. Zhonghua Er Bi Yan Hou Ke ZaZhi. 2000;35:26-8. [ Özet ]
7) Wei W, Heinze S, Gerstner DG, Walser SM, Twardella D, ReiterC, et al. Audiometric Notch and Extended High-FrequencyHearing Threshold Shift in Relation to Total Leisure Noise Exposure: An Exploratory Analysis. NoiseHealth. 2017;19: 263-269. [ Özet ]
8) Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, et al. The fate of outer hair cells after acoustic or ototoxic insults. HearRes 2006;218:20-9. [ Özet ]
9) Bird JE, Daudet N, Warchol ME, Gale JE. Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian innerear. J Neurosci 2010;30:12545-56. [ Özet ]
10) Parham K, Dyhrfjeld-Johnsen J. Outer Hair Cell Molecular Protein, Prestin, as a Serum Biomarker for Hearing Loss: Proof of Concept. OtolNeurotol. 2016;37:1217-22. [ Özet ]
11) Cao X, Fischer E, Gruschke O, Korvink JG, Hennig J, Maunder AM, et al. The noise factor of receiver coil matching networks in MRI. Magn Reson Imaging. 2017;37:252-9. [ Özet ]
12) Hurwitz R, Lane SR, Bell RA, Brant?Zawadzki MN. Acoustic analysis of gradient coil noise in MR imaging. Radiology 1989;173:545-48. [ Özet ]
13) Mollasadeghi A, Mehrparvar AH, Atighechi S, Davari MH, Shokouh P, Mostaghaci M, et al. Sensorineural hearing loss after magnetic resonance imaging. Case RepRadiol. 2013;2013:510258. [ Özet ]
14) Govindaraju R, Omar R, Rajagopalan R, Norlisah R, Kwan-Hoong N. Hearing loss after noise exposure. AurisNasusLarynx. 2011;38:519-22. [ Özet ]
15) Reavis KM, McMillan G, Austin D, Gallun F, Fausti SA, Gordon JS, et al. Distortion-product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear. 2011;32:61-74. [ Özet ]
16) Liba B, Naples J, Bezyk E, Campbell C, Mei M, Parham K. Changes in Serum Prestin Concentration After Exposure to Cisplatin. OtolNeurotol. 2017;38:e501-5. [ Özet ]
17) Wang Y, Hirose K, Liberman MC. Dynamics of Noise Induced Cellular Injury and Repair in the Mouse Cochlea. Journal of the Association for Research in Otolaryngology 2002;3:248-68. [ Özet ]
18) Revadi G, Rahmat O, Raman R, Norlisah R, Ng KH. Hearing loss after noise exposure. AurisNasusLarynx 2011;38:519-22. [ Özet ]
19) Hattori Y, Fukatsu H, Ishigaki T. Measurement and evaluation of acoustic noise of a 3 Tesla MR scanner. Nagoya J MedSci 2007;69:23-8. [ Özet ]
20) Wagner W, Staud I, Frank G, Dammann F, Plontke S, Plinkert PK. Noise in magnetic resonance imaging: no risk for sensorineural function but increased amplitude variability of otoacoustic emissions. Laryngoscope 2003;113:1216-23. [ Özet ]
21) Nakanishi N, Okamoto M, Nakamura K, Suzuki K, Tatara K. Cigarette smoking and risk for hearing impairment: A longitudinal study in Japanese male Office workers. J OccupEnvironMed, 2000;42:1045-9. [ Özet ]
22) Mizoue T, Miyamoto T, Shimizu T. Combined effect of smoking and occupational exposure to noise on hearing loss in steel factory workers. OccupEnviron Med,2003; 60:56-9. [ Özet ]
23) Paschoal CP, Azevedo MF. Cigarette smoking as a risk factor for auditory problems. Braz J Otorhinolaryngol, 2009;75:893-902. [ Özet ]
24) Mehrparvar AH, Mirmohammadi SJ, Ghoreyshi A, Mollasadeghi A, Loukzadeh Z. High-frequency audiometry: A means for early diagnosis of noise-induced hearing loss. NoiseHealth, 2011;13:402-6. [ Özet ]
25) Singh R, Saxena R, Varshney S. Early detection of noise induced hearing loss by using ultra high frequency audiometry. Internet J Otorhinolyngol, 2009;10:1-5.
26) Lopes AC, Otubo KA, Basso TC, Marinelli ÉJI, Lauris JRP. Occupational hearing loss: Tonal audiometry × High frequencies audiometry. IntArchOtolaryngol, 2009;3:293-302.
27) Campbell KCM, Le Prell CG. Drug-InducedOtotoxicity: Diagnosis and Monitoring. Drug Safety 2018; 41:451?64. [ Özet ]
28) Seixas NS, Neitzel R, Stover B, Sheppard L, Feeney P, Mills D, et al. 10-Year Prospective Study of Noise Exposure and HearingDamage among Construction Workers. Occupational and EnvironmentalMedicine 2012;69:643-50. [ Özet ]
29) Surovtseva EV, Johnston AH, Zhang W, Zhang Y, Kim A, Murakoshi M, et al. Prestin binding peptides as ligands for targeted polymersome mediated drug delivery to outer hair cells in the iner ear. Int J Pharm. 2012;15;424(1-2):121-7. [ Özet ]
30) He DZ, Lovas S, Ai Y, Li Y, Beisel KW. Prestin at year 14: progress and prospect. HearRes. 2014;311:25-35. [ Özet ]
31) Brummett RE, Talbot JM, Charuhas P. Potential hearing loss resulting from MR imaging. Radiology. 1988;169:539-40. [ Özet ]
32) Radomskij P, Schmidt MA, Heron CW, Prasher D. Effect of MRI noise on cochlear function. The Lancet. 2002;359:1485-6. [ Özet ]




