IS SNORING SOUND A CAUSE OF NOISE- INDUCED HEARING LOSS?
2Giresun University, Prof Dr A İlhan Özdemir Education and Research Hospital, Department of ENT, Giresun, Turkey
3Giresun University, Faculty of Medicine, Department of ENT, Giresun, Turkey
Summary
Purpose: The traumatic effects of intense snoring sound on the cochlea of snoring patient are still unclear. The aim of this study was to investigate whether snoring sound over 65 dB caused a perceivable hearing loss in snoring patients.Method: We included 72 patients with a pre- diagnosis of sleep apnea. To investigate the effects of snoring sound, we constituted two groups by reference to the max snoring sound level: lower snoring sound group (≤65 dB) and higher snoring sound group (>65 dB). We compared audiology parameters and the ratios of presence of a pure- tone threshold shift over 20 dB at every single frequency of both ears between these groups.
Results: Compared with lower snoring sound group, the median thresholds at 250, 500, 1000, 2000, 4000 and 8000 Hz; pure- tone averages and speech recognition threshold values were significantly higher in the right ears of high snoring sound group (p<0.001, p=0.004) as were in the left ears (p<0.001, p=0.005). The ratios of presence of a pure- tone threshold over 20 dB at 4000 Hz and 8000 Hz were significantly higher in high snoring sound group both for right (p=0.002 for 4000 Hz, p=0.001 for 8000 Hz) and left ears (p=0.002 for 4000 Hz, p<0.001 for 8000 Hz).
Conclusion: Snoring sound over 65 dB might negatively affect hearing functions in patients with sleep apnea, causing a perceivable hearing loss (threshold increase over 20 dB) at 4000 Hz and 8000 Hz.
Introduction
Snoring is the sound caused by the vibration of soft tissues due to upper airway resistance, and is the most common and major symptom of obstructive sleep apnea syndrome (OSA) [1]. Acoustic analysis of the snoring sound revealed a correlation between the characteristics of the snoring sound and polysomnography parameters [2]. Persistent snoring sound may not be an awakening trigger for the bed partner of a snoring patient [3] but the traumatic effects of intense snoring on the cochlea of snoring patient are still unclear. Snoring sound over 65 dB exposed for many years regarding the duration of the sleep disordered breathing may contribute to the hearing impairment in patients with OSA. Recent studies focusing on the association between hearing impairment and OSA suggested different mechanisms for hearing impairment including the damage of cochlear sensory epithelia and affected central auditory pathways [4-6]. However, to the best of our knowledge, a limited number of studies previously investigated the effects of snoring sound on hearing functions of snoring patients and their bed partners [7].The aim of this study was to investigate whether snoring sound over 65 dB caused a perceivable hearing loss in snoring patients, analyzing the changes in audiology parameters.
Methods
We provided the approval of the local ethical committee (IRB Number: 09/01/2019-01-07) for this prospective- clinical study and conducted the study in line with the dictates of the World Medical Association Declaration of Helsinki. We included 72 patients who were admitted to sleep disorders outpatient clinic. After taking a detailed medical history of all patients, we performed a comprehensive otolaryngologic and rhinologic examination, a pure- tone audiometry and a whole- night (type 1) polysomnography. We excluded the patients with the age of over 55 years (to avoid the effects of presbycusis) and the patients with any ear disease that might affect hearing functions including otitis media, perforated tympanic membrane, otosclerosis, Meniere"s disease, acoustic trauma, ototoxicity, and the patients with a history of otologic surgery. We used the Alice® 5 Diagnostic Sleep System (Respironics, Murrysville, PA, USA) linked to the Alice® Sleepware? software for whole -night PSG (Type 1) and we used fully -calibrated and well- maintained Orbiter 922- version 2 clinical audiometer (Madsen Electronics, Copenhagen, Denmark) for pure -tone audiometry. We noted the maximum snoring sound level (highest snoring sound level recorded during sleep), apnea- hypopnea index (AHI) and Desaturation index (DI) of each patient. Additionally, we noted the ages, genders, pure- tone averages (air conduction and bone conduction), pure- tone thresholds (PTT) (bone conduction) at 250, 500, 1000, 2000, 4000 Hz, and PTTs (air conduction) at 8000 Hz, speech discrimination scores (SDS) and speech recognition thresholds (SRT) of all patients. To investigate the effects of snoring sound, we constituted two groups by the reference of max snoring sound level: lower snoring sound (LSS) group (as control group) consisted of the patients with a max snoring sound level ?65 dB and higher snoring sound (HSS) group (as study group) consisted of the patients with a max snoring sound level >65 dB. Because it was the highest amount of sound that the cochleas of the patients were exposed during sleep, we used the highest snoring sound level measured (max snoring sound), to constitute the groups. We considered the term "noise" as intense sound over 65 dB, because sound intensity of normal conversation was reported to be under 65 dB [8]. Hence, the division limit of two groups was 65 dB in this study. To measure the max snoring sound level of the patients, we used an android smartphone (Samsung Galaxy A5 SM-A520, Samsung Electronics Ltd., Korea). To achieve an accurate measurement, we used the method previously described by Shin et al [9]. According to this method, we located the smartphone on the upper side of the shoulder without any special restriction, after a full calibration of the smartphone in silence. During patient monitoring, we ensured that all the sounds recorded during the sleep was snoring sound, and the records from other sources (sleep talking sound, outside sounds etc.) were excluded from the study. Firstly, we compared the pure- tone averages, PTTs, SRTs and SDSs of both ears between these groups. Then, we compared the ratios of presence of a PTT over 20 dB at every single frequency, between the groups. Additionally, we investigated the association between snoring sound intensity and severity of OSA. Finally, to facilitate the inter- study comparability, we utilized American Academy of Otolaryngology ? Head and Neck Surgery minimal reporting standard for reporting audiometric data [10] and constituted scatter diagrams of pretreatment hearing results.
Statistical Analysis
We presented the results as median (min-max). We investigated the distribution pattern of the data using Kolmogorov- Smirnov normality test. To compare AHIs, PTTs at 250, 500, 1000, 2000, 4000 and 8000 Hz; air-conduction and bone- conduction pure- tone averages; SRT values and SDS values of the groups, we used Mann Whitney- U test. To compare the ratios of presence of a PTT over 20 dB at every single frequency between the groups, we used chi- square test. To investigate the association of snoring sound intensity with AHI and DI, we used Spearman correlation test. For analysis of all data, we used SPSS 16.0 software for Windows (SPSS, Inc, Chicago, IL, USA). We considered a P value less than 0.05 statistically significant.
Results
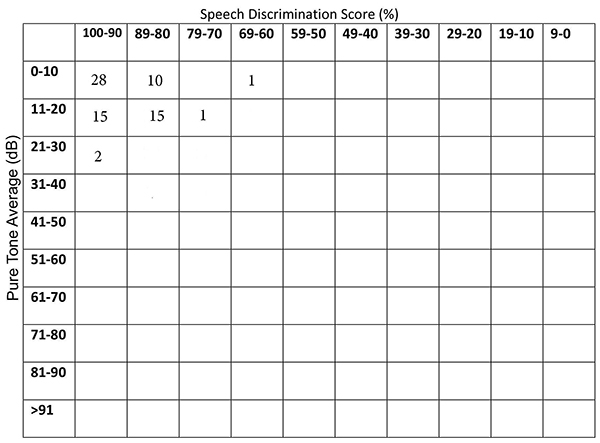
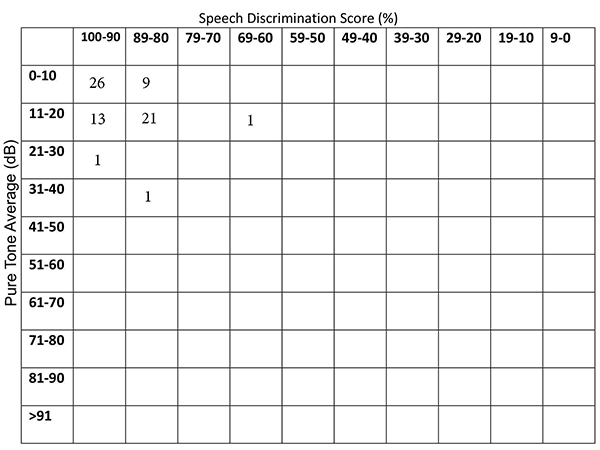
Seventy- two patients were eligible for this study. LSS group consisted of 37 patients (21 males and 16 females; mean age: 38 ±6 yrs) and HSS group consisted of 35 patients (24 males and 11 females; mean age: 40 ±7 yrs). The groups were age and gender- matched (p=0.19 p=0.3, respectively). The mean max snoring sound level of LSS group was 59 ±4 (50 -65) dB and the mean max snoring sound level of HSS group was 69 ±4 (66- 77) dB. The median AHI was significantly higher in HSS group [55 (21-129)] compared with LSS group [10.4 (1.4-39.1)] (P<0.001). Thus, intense snoring was associated with increased AHI.Fig. 1 represents the scatter diagram of the patient numbers according to pure tone averages (500-1000-2000-3000 Hz) and SDSs of the right ears [10]. Fig. 2 represents the scatter diagram of the patient numbers according pure tone averages (500-1000-2000-3000 Hz) and SDSs of the left ears [10].
 Büyütmek İçin Tıklayın |
Figure 1: The scatter diagram of the patient numbers according to pure tone averages (500-1000-2000-3000 Hz) and SDSs of the right ears. |
 Büyütmek İçin Tıklayın |
Figure 2: The scatter diagram of the patient numbers according to pure tone averages (500-1000-2000-3000 Hz) and SDSs of the left ears. |
Compared with LSS group, the median PTTs at 250, 500, 1000, 2000, 4000 and 8000 Hz; median air- conduction and bone- conduction pure- tone averages; and median SRT values were significantly higher in the right ears of HSS group (p<0.001, p=0.004) (Table 1) as were in the left ears of HSS group (p<0.001, p=0.005) (Table 2). Additionally, compared with LSS group, median SDS value was significantly lower in the right ears of HSS group (p=0.011) (Table 1), as was in the left ears of HSS group (0.017) (Table 2). Thus, snoring sound over 65 dB was significantly associated with impairment of all hearing functions.
The ratios of presence of a PTT over 20 dB at every single frequency were presented in Table 3 for the right ears and in Table 4 for the left ears. For the right ears, the ratio of presence of a PTT over 20 dB at 4000 Hz was 28.6% (10 patients) in HSS group and 2.7% (1 patients) in LSS group, thus significantly greater in HSS group (X 2= 9.29, p= 0.002). The ratio of presence of a PTT over 20 dB at 8000 Hz was 37.1% (13 patients) in HSS group and 5.4% (2 patients) in LSS group, again, significantly greater in HSS group (X 2= 10.98, p= 0.001).
For the left ears, the ratio of presence of a PTT over 20 dB at 4000 Hz was 28.6% (10 patients) in HSS group and 2.7% (1 patients) in LSS group, thus significantly greater in HSS group (X 2= 9.29, p= 0.002). The ratio of presence of a PTT over 20 dB at 8000 Hz was 45.7% (16 patients) in HSS group and 5.4% (2 patients) in LSS group, again, significantly greater in HSS group (X 2= 15.58, p< 0.001). Comparisons revealed that snoring sound affected all hearing functions but its effects on hearing functions were found to be significant particularly at 4000 Hz and 8000 Hz frequencies, causing a perceivable threshold increase (over 20dB).
Spearman correlation test revealed that snoring sound level was positively, significantly, and strongly correlated with AHI (rho: 0.87, p<0.001) and DI (rho: 0.83, p<0.001). Thus, snoring sound intensity was associated with the severity of OSA.
Discussion
The effects of the noise exposure on hearing functions may vary due to intensity of the noise, exposure duration and exposure pattern [11]. Overexposure to hazardous levels of noise may be defined as noise- induced hearing loss (NIHL). A common type of NIHL is occupational hearing loss. In recent studies, NIHL of different worker groups like textile workers and military pilots were reported [12,13]. Al- Omari et al. reported that the pilots with a higher flying hours had a significantly greater risk of NIHL [12]. Thus, exposure time has a significant clinical implication as does intensity of the noise. Although snoring sound is not an occupational noise source and not intense as occupational sounds, it can be considered as a noise source for OSA patients causing a NIHL because of long exposure durations regarding the duration of disease. In our previous publication, we reported the cross- sectional association between the severity of OSA and hearing loss [14]. Although the causative mechanism of hearing loss in OSA patients is not clear, snoring sound might be a contributing factor for hearing loss, because we found a significant correlation between the snoring sound intensity and the severity of OSA in this study. Snoring sound may affect the cochleas of both snoring patients and their bed partners but the most significant negative effect is expected to occur in the patient him/herself, because the harsh sound produced from resistant upper airway directly reaches to the cochlea of the patient by bone-way conduction as well as airway conduction. However, snoring sound might affect the quality of life of the bed partner [3], as well as the patient [15,16]. Sardesai et al. reported a relationship between snoring and hearing loss, and they also suggested further investigations addressing this topic [7]. In this study, we aimed to reveal the negative effects of snoring sound on the cochleas of snoring patients regardless the severity of the disease. We found a perceivable hearing loss particularly at higher frequencies in patients with snoring sound over 65 dB.OSA is a multisystem disease characterized by repetitive episodes of complete or partial upper airway obstruction. Upper airway obstruction in OSA patients results in chronic intermittent hypoxemia and reduced blood oxygen saturation [17]. It has many negative effects on multiple systems including cochlear and vestibular systems [4,6,18,19]. The main mechanism of hearing loss in OSA is still a matter of debate. However, snoring sound might be a contributing factor of hearing loss in OSA patients. In our study, we constituted two groups by reference to the snoring sound level and compared the audiology parameters of two groups. We set the snoring sound level measurement equipment in the sleep laboratory along with the polysomnography. Because our groups were age and gender matched, the only decoupling factor between the study group and the control group was snoring sound level. We thought that long- term exposure to snoring sound over 65 dB might contribute to the hearing impairment in OSA patients. However, Cathcart et al. reported a night- to- night variety in snoring sound intensity [20]. Although this study was a one- night study and lack of multi- night measurements of snoring sound levels, all participants in our study group had the evidence of measured snoring sound level over 65 dB.
In this study, we found that snoring sound over 65 dB had significant effects on all hearing functions. However, the median hearing thresholds of HSS group in many frequencies were still under 20 dB. Because, the shifts over 20 dB might be considered as a perceivable hearing loss, the effects of snoring on the quality of life was controversial. To investigate the effects of snoring sound on the quality of life regarding the threshold shifts over 20 dB, we compared the ratios of presence of a PTT over 20 dB at every single frequency, between the groups. Our results showed that snoring sound perceivably affected the pure tone thresholds at 4000Hz and 8000Hz. Thus, we can hypothesize that snoring sound significantly affects high frequency hearing functions in consistent with the other types of NIHL. In addition, a significant difference between SDSs of two groups was evident in our study.
In a study of analyzed snoring sounds, Herzog et al. reported a positive correlation between the peak intensity of snoring sound and the AHI [2]. In addition to the effects on cochlear functions, the snoring may have another clinical implication since it might be associated with the severity of OSA. Supporting this contention was our finding that snoring sound level was significantly and strongly correlated both with AHI and DI. This result suggested that severe OSAS patients had a higher snoring sound level, with a higher risk of NIHL.
Although represents a significant association between snoring sound intensity and hearing loss, this study has several limitations. The major limitation of the study was that our groups were heterogenous in terms of OSA severity, because snoring sound level was positively correlated with OSA severity. Thus, homogenizing our groups in terms of OSA severity was not possible when splitting the groups by snoring sound level. Another limitation was the limited number of the participants. In addition, our data was lack of the values of the bed partners of the patients. Thus, future studies including the bed partners of the patients are needed.
Conclusion
In conclusion, snoring sound over 65 dB might negatively affect hearing functions in patients with OSA as a noise source. Effects of snoring sound on hearing functions were significant particularly at 4000 Hz and 8000 Hz frequencies, causing a perceivable threshold-increase over 20 dB.Conflict of interest: The authors declare no conflict of interest.
Funding: No funding was received for this study.
Reference
1) Koo SK, Kwon SB, Kim YJ, Moon JIS, Kim YJ, Jung SH. Acoustic analysis of snoring sounds recorded with a smartphone according to obstruction site in OSAS patients. Eur Arch Otorhinolaryngol 2017; 274: 1735-1740. [ Özet ]
2) Herzog M, Schmidt A, Bremert T, Herzog B, Hosemann W, Kaftan H. Analysed snoring sounds correlate to obstructive sleep disordered breathing. Eur Arch Otorhinolaryngol 2008; 265: 105-113. [ Özet ]
3) Blumen MB, Quera Salva MA, Vaugier I, Leroux K, d'Ortho MP, Barbot F, Chabolle F, Lofaso F. Is snoring intensity responsible for the sleep partner's poor quality of sleep? Sleep Breath 2012; 16: 903-907. [ Özet ]
4) Chopra A, Jung M, Kaplan RC, Appel DW, Dinces EA, Dhar S, Zee PC, Gonzalez F, 2nd, Lee DJ, Ramos AR, Hoffman HJ, Redline S, Cruickshanks KJ, Shah NA. Sleep Apnea Is Associated with Hearing Impairment: The Hispanic Community Health Study/Study of Latinos. J Clin Sleep Med 2016; 12: 719-726. [ Özet ]
5) Iriz A, Duzlu M, Kokturk O, Kemaloglu YK, Eravci FC, Kuukunal IS, Karamert R. The effect of obstructive sleep apnea syndrome on the central auditory system. Turk J Med Sci 2018; 48: 5-9. [ Özet ]
6) Seo YJ, Ju HM, Lee SH, Kwak SH, Kang MJ, Yoon JH, Kim CH, Cho HJ. Damage of Inner Ear Sensory Hair Cells via Mitochondrial Loss in a Murine Model of Sleep Apnea With Chronic Intermittent Hypoxia. Sleep 2017; 40: 1-7. [ Özet ]
7) Sardesai MG, Tan AK, Fitzpatrick M. Noise-induced hearing loss in snorers and their bed partners. J Otolaryngol 2003; 32: 141-145. [ Özet ]
8) Kemaloglu Y. Gürültü ve Gürültüye Bağlı İşitme Kayıpları. In: Onerci M (ed) Kulak Burun Boğaz Baş Boyun Cerrahisi. Ankara: Matsa Basımevi, 2016:449- 460.
9) Shin H, Cho J. Unconstrained snoring detection using a smartphone during ordinary sleep. BioMedical Engineering OnLine 2014; 13: 116 (PMID).
10) Gurgel RK, Jackler RK, Dobie RA, Popelka GR. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 2012; 147: 803-807. [ Özet ]
11) Gates G, Clark W. Occupational Hearing Loss. In: Lalwani A (ed) Current Diagnosis & Treatment in Otolaryngology-Head & Neck Surgery, 3rd Edition. New York: McGraw- Hill Companies, 2012:747-759.
12) Al-Omari AS, Al-Khalaf HM, Hussien NFM. Association of Flying Time with Hearing Loss in Military Pilots. Saudi J Med Med Sci 2018; 6: 155-159. [ Özet ]
13) Shahid A, Jamali T, Kadir MM. Noise Induced Hearing Loss among an Occupational Group of Textile Workers in Karachi, Pakistan. Occup Med Health Aff 2018; 6: 1-15. [ Özet ]
14) Kayabasi S, Hizli O, Yildirim G. The association between obstructive sleep apnea and hearing loss: a cross-sectional analysis. Eur Arch Otorhinolaryngol 2019. [ Özet ]
15) Nakano H, Furukawa T, Nishima S. Relationship between snoring sound intensity and sleepiness in patients with obstructive sleep apnea. J Clin Sleep Med 2008; 4: 551-556. [ Özet ]
16) Kalchiem-Dekel O, Westreich R, Regev A, Novack V, Goldberg M, Maimon N. Snoring intensity and excessive daytime sleepiness in subjects without obstructive sleep apnea. Laryngoscope 2016; 126: 1696-1701. [ Özet ]
17) AASM. International Classification of Sleep Disorders, 3rd ed, American Academy of Sleep Medicine. Darien, IL, 2014.
18) Hizli O, Ozcan M, Unal A. Evaluation of comorbidities in patients with OSAS and simple snoring. ScientificWorldJournal 2013; 1-4. [ Özet ]
19) Kayabasi S, Iriz A, Cayonu M, Cengiz B, Acar A, Boynuegri S, Mujdeci B, Eryilmaz A. Vestibular functions were found to be impaired in patients with moderate-to-severe obstructive sleep apnea. Laryngoscope 2015; 125: 1244-1248. [ Özet ]
20) Cathcart RA, Hamilton DW, Drinnan MJ, Gibson GJ, Wilson JA. Night-to-night variation in snoring sound severity: one night studies are not reliable. Clin Otolaryngol 2010; 35: 198-203. [ Özet ]




