IS THERE ANY PROGNOSTIC INFLUENCE OF TUMOR-ASSOCIATED TISSUE EOSINOPHILIA IN LIP CANCER?
2Numune Training and Research Hospital, Pathology Department, Adana, TURKEY
3Aşkım Tüfekçi Hospital. Otorhinolaryngology and Head and Neck Surgery Department, Adana, TURKEY
Summary
Purpose: Many prognostic factors have been identified for squamous cell carcinoma (SCC) occurring in the oral cavity, one of which is tumor-associated tissue eosinophilia (TATE). The aim of this study was to investigate the effects of TATE on prognosis and its relationship with clinical and pathological variables only in lip cancers in order to create a homogenous study group.Materials and Methods: This retrospective study included 31 consecutive patients with a diagnosis of lip cancer who were admitted to Health and Science University Adana City Hospital, Ear-Nose-Throat Clinic from September 2010 to September 2016 and treated with surgery.
Results: When the relationship of TATE with other variables was investigated, a significant relationship was found only with smoking. Smokers had higher mild intratumoral TATE. There was no significant relationship of tumor grade, recurrence, neck dissection, blood eosinophilia, perineural invasion, lymph node metastasis, differentiation level, and invasion depth with peritumoral-intratumoral TATE. No statistically significant relationship between peritumoral TATE and smoking was found.
Conclusion: The predictors of recurrence or prognosis at the time of initial diagnosis are inadequate in lip cancer. It is promising to evaluate eosinophilia as a possible diagnostic tool, which can already be assessed on hematoxylin and eosin (H&E)-stained tumor slides. For this reason, it may be better to include findings on eosinophilia in the pathology report.
Introduction
Lip cancer is the most commonly occurring cancer in the oral cavity, accounting for 25%30% of all cases, and it is the second most common type of cancer in the headneck region after cutaneous cancers [1]. Many prognostic factors have been identified for squamous cell carcinomas (SCCs) occurring in the oral cavity, one of which is tumor-associated tissue eosinophilia (TATE). TATE is defined as the presence of eosinophils in peritumoral or intratumoral tissues [2]. It was reported in the headneck region for the first time by Lowe et al. [3]. There are conflicting studies suggesting TATE as a good prognostic factor [4], a poor prognostic factor [5], and an ineffective factor[6]. These studies have investigated the relationship of TATE with various variables.Eosinophils are granulocytes found in the bloodstream, which originate from the bone marrow. The level of eosinophils increases in parasitic and allergic conditions, and they participate in tissue remodeling and immune responses [7]. Recently, their antitumor effect has been revealed. Eosinophils contain specific granules, such as eosinophil cationic protein (ECP), major basic protein (MBP), granulocyte-macrophage colony-stimulating factor (GMSF), interleukin (IL)-3, IL-5, tumor necrosis factor (TNF)-alpha, transforming growth factor (TGF)-alpha, and transforming growth factor (TGF)-beta. Such a variety of eosinophil-derived mediators allows eosinophils to function as antitumor effector cells in neoplastic lesions [8,9]. These cells are found in vast numbers in SCC of the oral cavity [2], cervix, lower colon, and anus [10].
The present study included only lip cancers in order to create a homogenous study group. We investigated the effects of TATE on prognosis and its relationship with clinical and pathological variables.
Methods
Assessment of patientsA total of 31 patients (28 males, 3 females), who were admitted to the Ear, Nose, and Throat Clinics of the University of Health Sciences, Adana Numune Research and Training Hospital, from September 2010 to September 2016, with a complaint of a lip mass and who were operated on for SCC of the lip, were included in the study. Patients who underwent surgery as the initial treatment, those for whom pathology slides were available, and those who underwent clinical follow-up for SCC were also included in the study. Patients who previously underwent chemotherapy or radiotherapy, those who did not provide consent for surgery, those with another primary tumor or unresectable tumor, those with a tumor at another site in the body, those with distant metastasis, those who did not have TATE, and those who did not continue follow-up were excluded from the study. Age, gender, smoking status, alcohol use, tumornodemetastasis, treatment, recurrence, and survival were recorded.
Study Design and TATE Analysis
This study was designed as a retrospective clinical trial. All the patients provided written informed consent, the study was according to principles of Helsinki Declaration and the study was approved by the local ethics committee of Adana Numune Research and Training Hospital (201733). The patients underwent surgery in a wide spectrum of procedures from wedge resection to neck dissection combined with reconstructive surgery. For the study, 10% formalin-fixed paraffin blocks were obtained from the archives of the Department of Pathology. Pathological examination was conducted by a single physician blinded to the patients clinical information at the Department of Pathology. In the hematoxylin and eosin (H&E)-stained sections, eosinophils in the epithelium of tumor (peritumoral) and intratumoral stroma (intratumoral) were counted by light microscope in 10 continuous high-power fields (HPFs) (x400 magnification) in a zigzag fashion as described by Goldsmith: 510 eosinophils/HPF: + 1; 1020 eosinophils/HPF: + 2; 2030 eosinophils/PF: + 3; and > 30 eosinophils/HPF: + 4, in which 0 to + 1 indicates absent/mild, + 2 and + 3 indicate moderate, and + 4 indicates intense eosinophilia. Eosinophils were defined as cells with lobulated nuclei and red cytoplasmic granules. Particular care was taken to not count erythrocytes superimposed with mononuclear or polymorphonuclear inflammatory cells in fields overlapping with blood vessels. Cells considered to be erythrocytes were not included. TATE was assessed in the intratumoral field, as well as in the peritumoral area. Infiltration pattern and tumor morphology were examined. The patients were followed up from surgery until the last day of the study or death.
Statistical Analysis
The data obtained from this study were analyzed using IBM SPSS Statistics ver. 20 software package (IBM, Armonk, NY, USA). The Shapiro-Wilk test was used to investigate normality of the variables because of the unit numbers. When the results were interpreted, the significance level was set at 0.05, with p < 0.05 values indicating a significant difference and p > 0.05 values showing no significant difference. Because the variables did not show a normal distribution, the Kruskal-Wallis H test was used for analyzing the differences between the groups. In the cases of significant differences obtained from the Kruskal-Wallis H test, the post-hoc multiple comparison test was used to determine the groups causing the differences.
Results
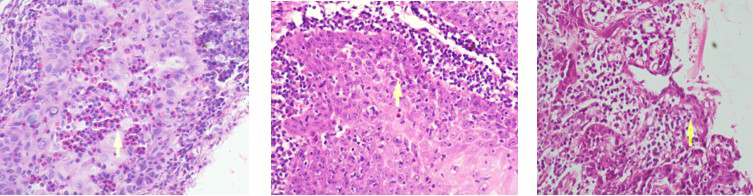
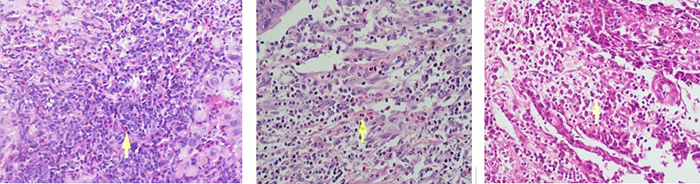
There was a predominance of males (90.3%) in the 31 patients examined with SCC of the lip. Their ages ranged from 30 to 91 years (mean age: 60 years). Of these patients, 64.5% were older than 65 years, 58.1% were smokers, and 16.1% were alcohol consumers. In terms of tumor excision, 19.4% underwent unilateral and 32.3% underwent bilateral neck dissection. Moreover, 9.6% of the patients had higher blood eosinophilia. A total of 12 (38,7%) patients were at the T1, 16(51,6%) patients were at the T2, and three(9,7%) patients were at the T3 stage. Two(6,45%) patients were at the N2 stage, and the remaining patients((93,54%) were at the N0 stage. Three patients had perineural invasion, whereas no patients had vascular invasion. 12 (38.7%) patients had well-differentiated tumors, 16 (51.6%) patients had moderately differentiated tumors, and 3 (9.6%) patients had poorly differentiated tumors. Of the five patients who developed nodal metastasis, two underwent chemoradiotherapy, and three underwent radiotherapy alone (Table 1). The patients were followed up for 4-72 months. Of the patients with epithelial TATE: 2 had intense TATE (Figure 1a), 14 had moderate TATE (Figure 1b), and 15 had mild TATE (Figure 1c). Among the patients with stromal TATE: seven had intense (Figure 2a), 15 had moderate (Figure 2b), and nine had mild TATE (Figure 2c) (Table 2).Table 1: Main clinical parameters of 31 patients with SCC of the lip.
Table 2: Number of patients of TATE type and degree. TATE, tumor-associated tissue eosinophilia
 Büyütmek İçin Tıklayın |
Figure 1: Epithelial intense(a), moderate(b), mild(c) eosinophilia respectively (x400 magnification) |
 Büyütmek İçin Tıklayın |
Figure 2: Stromal intense(a), moderate(b), mild(c) eosinophilia respectively (x400 magnification) |
When the relationship of TATE with other variables was investigated, a significant relationship was found only with smoking. Smokers had higher mild intratumoral TATE.
There was no significant relationship of age, gender, tumor grade, recurrence, neck dissection, blood eosinophilia, perineural invasion, lymph node metastasis, differentiation level, and invasion depth with peritumoral and intratumoral TATE. There was a statistically significant relationship between smoking and intratumoral TATE (p = 0.008). When the proportions were compared, using the paired comparison method to investigate the source of this relationship, the number of patients with mild TATE was significantly higher among smokers (p = 0.005). There was no statistically significant relationship between peritumoral TATE and smoking (p = 0.455) (Table 3).
Discussion
Even though much is known about the association between the infiltration of eosinophils and neoplasms, little is known about how chemokines affect the differentiation and migration of eosinophils is increasing. Many kinds of chemokines are involved in the migration and differentiation of eosinophils [11-15]. Lymphocyte-derived eosinophilotactic cytokines, such as lymphocyte chemoattractant factor, IL-2, IL-3, IL-4, IL-5, and regulated upon activation, normal T cells expressed and secreted were found to be associated with TATE. Moreover, vascular cell adhesion molecule 1 expression was found to be increased, and eosinophils were found to migrate to endothelium with the increasing levels of these cytokines [6,16]. MBP [17,18] and ECP [18] released from eosinophils possess cytotoxic effects and exhibit cytotoxic activity on the tumor. The results obtained from immunotherapeutic approaches in epithelial tumor patients show that the accumulation of eosinophils has a clear mechanism. The efficacy of treatment with recombinant IL-2 in advanced cancers was related to more peritumoral eosinophilia [19]. The eosinophil-dependent antitumoral mechanism of IL-4 was also supported by animal studies [20].The predictors of recurrence or prognosis at the time of initial diagnosis are inadequate in oral cancers. Various studies have investigated the possible relationship between TATE and various tumors. Along with studies describing TATE as a good prognostic factor or poor prognostic factor, there are studies in the literature suggesting that TATE cannot predict tumor prognosis. The present study also concludes that TATE has no effect on survival and locoregional metastasis. Dorta et al. suggested that better prognosis could be made by increasing the TATE grade in SCC of the oral cavity [2]. Landman et al. also suggested favorable effects of TATE on prognosis for these tumors [10]. Nagaraju et al. reported intense TATE in patients with locoregional recurrence. They also found higher eosinophil counts in recurrent tumor tissue when compared with primary tumors [11]. Alrawi et al. stated that the rate of stromal invasion and recurrence increases with increasing TATE grade [12]. Oliveira et al. found a positive relationship between TATE and lymph node metastasis in patients with N0 stage disease and emphasized that TATE could predict lymph node metastasis [13]. Falconieri et al. reported a significant relationship between TATE grade and metastatic lymph node [14]. In the present study, lymph node metastasis and TATE were found to be unrelated to each other. Because of the fact that many studies have found high eosinophil counts in invasive tumors, studies in the literature have suggested the use of tissue eosinophilia as a histopathological marker related to stromal invasion [15]. Tadbir et al. investigated the rate of TATE in three areas of a tumor. They found a higher grade of TATE in the invasive tumor site compared with that in other intratumoral areas and stroma under the epithelium; however, they reported no relationship between TATE and histopathological values and locoregional metastasis [21]. Said et al. reported a highly significant relationship between tissue eosinophilia and stromal invasion in patients with SCC of the larynx [15]. In the literature, TATE was reported in 22%89% of patients with SCC of the head and neck; it was 81.5% in this study. The present study evaluated intratumoral and peritumoral TATE grades and investigated their relationship with survival parameters. There was no significant relationship between survival parameters and peritumoral-intratumoral TATE.
TATE grade was found to be lower in normal oral mucosa compared with that in oral leukoplakia, and TATE in leukoplakia increased in the presence of dysplasia. This finding could be related to the smoking status [19]. In our study, mild intratumoral TATE is significantly higher in smokers out of touch with survival. Another study found absent or mild TATE in oral verrucose SCC and intense TATE in stage 3 or 4 tumors, but no relationship with survival [22].
In various studies, tissue eosinophilia count was determined using different systems. The classification by Goldsmith [20], which was mentioned above, was used for this study.
In a study conducted on patients with uterine SCC, TATE was shown to increase with the increasing level of blood eosinophilia. TATE was suggested as a good prognostic factor in another study conducted on patients with SCC of the cervix, and blood eosinophilia was concluded to have unfavorable effects on prognosis [23]. The present study found no relationship between blood eosinophilia and tissue eosinophilia.
In the present study which evaluated SCC of the lip instead of SCC of the oral cavity in which the rate of occult metastasis is reported to exist in a wide range, no significant relationship was found between TATE and survival parameters.
This study has some limitations. Although lip cancer is the most common type of tumor among oral SCCs, it is detected less often than other diseases. Thus, the small number of patients is the primary limitation of the present study. Investigation of this subject in a larger series of patients with SCCs in different sites of the oral cavity would clarify the data in the literature.
In conclusion, we found a statistically significant relationship between smoking and intratumoral TATE, whereas there was no statistically significant relationship between stromal TATE and smoking. Eosinophilia can already be assessed on H&E-stained tumor slides. For this reason, it may be better to mention eosinophilia in pathology reporting. Although it is considered to be an important predictor in oral cavity tumors, this is yet to be clarified in the literature. Further studies on peritumoral eosinophilia and studies on the eosinophils in tumor tissues of the lip are needed for a more detailed understanding of the immunologic characteristics of lip cancer.
Acknowledgements
This study received no funding. The authors declare that they have no conflicts of interests in regard to this work. All procedures performed in this study involving human participants were conducted in accordance with the ethical standards of the Institutional and National Research Committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Ethical approval was provided by the Ethics Committee of the University of Health Sciences, Adana Numune Research and Training Hospital. The ethical approval number is ANEAH EK-2017/33.
Reference
1) Moore S, Johnson N, Pierce A, Wilson D. The epidemiology of lip cancer: a review of global incidence and aetiology. Oral Dis 1999; 5: 185-195. [ Özet ]
2) Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology 2002; 41: 152-157. [ Özet ]
3) Lowe D, Fletcher C, Shaw M, McKee PH. Eosinophil infiltration in keratoacanthoma and squamous cell carcinoma of the skin. Histopathology 1984; 8: 619-625. [ Özet ]
4) Thompson A, Bradley P, Griffin N. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am J Surg 1994; 168: 469-471. [ Özet ]
5) Horiuchi K, Mishima K, Ohsawa M, Sugimura M, Aozasa K. Prognostic factors for well-differentiated squamous cell carcinoma in the oral cavity with emphasis on immunohistochemical evaluation. J Surg Oncol 1993; 53: 92-96. [ Özet ]
6) Sassler A, McClatchey K, Wolf G, Fisher SG. Eosinophilic infiltration in advanced laryngeal squamous cell carcinoma. Laryngoscope 1995; 105: 413-416. [ Özet ]
7) Davoine F, Sim A, Tang C, Fisher S, Ethier C, Puttagunta L, et al. Eosinophils in human oral squamous carcinoma; role of prostaglandin D2. J Inflamm (Lond) 2013; 10:4. 8) Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, et al . Human eosinophils exert TNFalpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells . J Immunol 2010; 185: 7443-51. [ Özet ]
9) Gleich G. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 2000; 105: 651-663. [ Özet ]
10) Lorena SC, Dorta RG, Landman G, Nonogaki S, Oliveira DT. Morphometric analysis of the tumor associated tissue eosinophilia in the oral squamous cell carcinoma using different staining techniques. Histol Histopathol 2003; 18: 709-713. [ Özet ]
12) Alrawi SJ, Tan D, Stoler DL, Dayton M, Anderson GR, Mojica P, et al. Tissue eosinophilic infiltration: a useful marker for assessing stromal invasion, survival and locoregional recurrence in head and neck squamous neoplasia. Cancer J 2005; 11: 217-225. [ Özet ]
13) Oliveira DT, Biassi TP, Faustino SE, Carvalho AL, Landman G, Kowalski LP. Eosinophils may predict occult lymph node metastasis in early oral cancer. Clin Oral Investig 2012; 16: 1523-1528. [ Özet ]
14) Falconieri G, Luna MA, Pizzolitto S, DeMaglio G, Angione V, Rocco M. Eosinophil-rich squamous carcinoma of the oral cavity: a study of 13 cases and delineation of a possible new microscopic entity. Ann Diagn Pathol 2008; 12: 322-327. [ Özet ]
15) Said M, Wiseman S, Yang J, Alrawi S, Douglas W, Cheney R , et al. Tissue eosinophilia: a morphologic marker for assessing stromal invasion in laryngeal squamous neoplasms. BMC Clin Pathol 2005;5:1. [ Özet ]
16) Leighton SE, Teo JG, Leung SF, Cheung AY, Lee JC, van Hasselt CA. Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer 1996; 77: 436-440. [ Özet ]
17) Deron PH, Goossens A, Halama AR. Tumour-associated tissue eosinophilia in head and neck squamous-cell carcinom. ORL J Otorhinolaryngol Relat Spec 1996; 58: 167-170. [ Özet ]
18) Tepper RI. The eosinophil-mediated antitumor activity of interleukin-4. J Allergy Clin Immunol 1994; 94: 1225-1231. [ Özet ]
19) Madhura MG, Gajalakshmi S, Kumar BV, Suma S, Sarita Y, Shweta RD. Role of tissue eosinophils in oral leukoplakia: a pilot study. J Oral Maxillofac Pathol 2015; 19: 286-290. [ Özet ]
20) Goldsmith MM, Belchis DA, Cresson DH, Merritt WD 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg 1992; 106: 27-33. [ Özet ]
21) Tadbir AA, Ashraf MJ, Sardari Y. Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg 2009;20:287-289. [ Özet ]
22) Tostes Oliveira D, Tjioe KC, Assao A, Sita Faustino SE, Lopes Carvalho A, Landman G, Kowalski LP. Tissue eosinophilia and its association with tumoral invasion of oral cancer. Int J Surg Pathol 2009; 17: 244-249. [ Özet ]
23) Alkhabuli JO, High AS. Significance of eosinophil counting in tumor associated tissue eosinophilia (TATE). Oral Oncol 2006; 42: 849-850. [ Özet ]




