INTERFERENCE BETWEEN HELICOBACTER PYLORI AND SQUAMOUS CELL CARCINOMA OF THE LARYNX: HISTOPATHOLOGIC AND SEROLOGIC STUDY
2Ankara Yüksek İhtisas Hastanesi, Patoloji, Ankara, Türkiye
3Ankara Hastanesi, Mikrobiyoloji, Ankara, Türkiye
Summary
The presence of IgG antibodies against H. pylori antigens in the sera of 30 patients who are diagnosed with squamous cell carcinoma of the larynx were tested using ELISA technique. The presence of H. pylori in the larynx specimens was also investigated microscopically. At least two fresh histopathologic samples were taken each from tumoral and non-tumoral tissues. Biopsies were stained with haematoxylin and eosin, and giemsa. The incidence of seropositivity was found to be 60%. H. pylori was detected in 12 (40%) patients' histopathologic examinations. In 6(%20) patients, bacteria were found in both normal and tumoral tissues, and in 6 patients were found in only tumor tissue. H. pylori was detected in specimens of 50% of the seropositive patients. Although we detected H. pylori in laryngeal specimens, we could not explain its role on carcinogenesis. Further studies to determine the role of HP as a carcinogen and its relationship with other environmental factors in laryngeal cancer are warranted.Introduction
Helicobacter species are, gram-negative bacteria, strict microaerophiles with spiral or helical morphology and will also grow in air with increased CO2 content. The optimum temperature for isolation is 35°C to 37°C, although some strains will grow at 42°C. High humidity has also been found to favor growth. The characteristic gram stain (small, curved and slightly plump bacilli) and positive reactions for catalase, oxidase and urease provide identification. Human, monkeys and cats are its hosts. Helicobacter pylori (H. pylori) can cause chronic infection. H. pylori gastritis is widespread in many countries in the world and also may be one of the most common chronic human infections. The prevalence of H. pylori infection shows marked geographical and regional differences all over the world (Table 1)[1,2]. It is reported that the incidence ranges from 60% to 84% of the Turkish population[3].Table 1: Seroprevalence rates from countries.
The international Agency for Research on Cancer (IARC), a working group of World Health Organization, acknowledged H. pylori as a group 1 (definite) carcinogen of gastric carcinoma in 1994[4]. The larynx is a part of aerodigestive system and in continuity with the esophagus and the stomach. Several studies have reported the isolation of this bacterium both from dental plaque and saliva. The role of chronic infection in squamous cell carcinoma of the larynx (LSCC) has not been specially addressed[5].
We previously reported an etiologic role of H. pylori infection on development of LSCC by serologic findings[6]. The purpose of this study is to determine interference between LSCC and serology of HP and active HP infection in the larynx specimen.
Methods
Serum samples and specimen biopsies from 30 patients with LSCC were evaluated. All of the subjects were male who smoked; their age distribution was 40 to 84 years (average, 57.9 years). None of the patients had gastrointestinal complaints (ulcer-like pain, nausea, vomiting, gastritis, duodenal or gastric ulcus) currently. Serum samples obtained from patients were maintained at -70°C and analyzed. Titers of anti-H. pylori IgG were assayed by a serum enzyme linked immunoabsorbant assay (ELISA). ELISA (RADIM, Italy) is a serological diagnostic test that is used to detect antibodies that are formed against certain antigens by binding an enzyme horseradish peroxidase. It has a sensitivity of 96% and specificity of 93%[3]. Serum optical density that was higher than 30 UR/ml seropositive, other values that were lower than 15 UR/ml were accepted as negative.Fresh histopathologic samples was taken each from tumoral tissue and non-tumoral mucosa at least two biopsy specimens. Biopsies were stained with haematoxylin and eosin and giemsa.
The study was conducted in compliance with the "Ethical principles for medical research involving human subjects" of Helsinki Declaration.
Results
The seropositivity rate was 60% (18/30). In 40% of patients, H. pylori was found in the laryngeal specimens (12/30). H. pylori was found 20% of patients in each normal mucosa and tumoral tissue (6/30). We detected bacteria in specimens of 50% of the seropositive patients (9/18) (Figure 1).
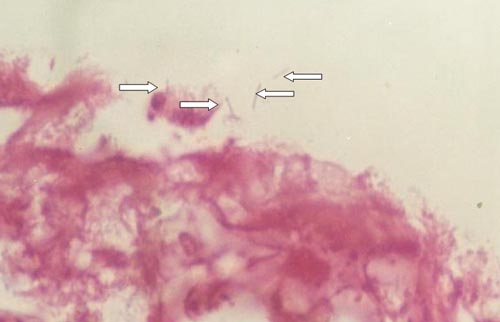 Büyütmek İçin Tıklayın |
Figure 1: Helicobacter pylori in the squamous cell carcinoma of the larynx. HE x 1000 |
We histopatologically determined that chronic inflammation shown by lymphocyte infiltration is present in all HP + laryngeal carcinoma tissiu (Figure 2) and lymphoid aggregates are present 83% of H. pylori positive larynx biopsy specimens (10/12) (Figure 3).
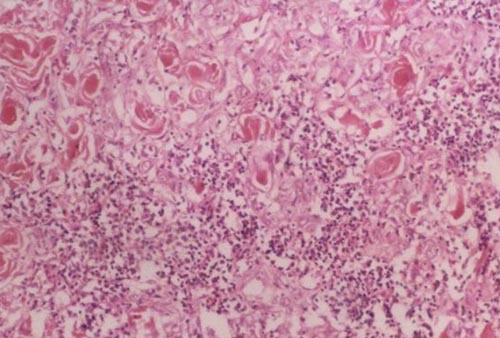 Büyütmek İçin Tıklayın |
Figure 2: Polymorphonuclear leucocytes and lymphoplasmocyte infiltration (HE x 100). |
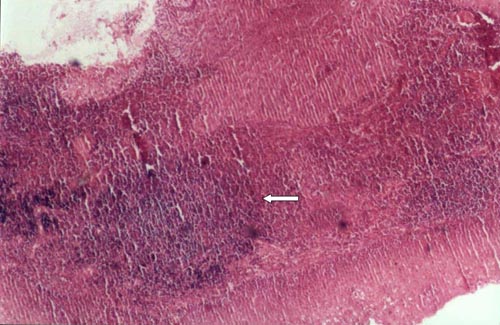 Büyütmek İçin Tıklayın |
Figure 3: Lymphoid aggregates are present in HP positive in specimens (HE x 40). |
Discussion
H. pylori infection is now recognized as a serious transmissible infectious disease and half of the world population is infected with H. pylori with the majority of infected individuals thought to remain asymptomatic throughout their lives. The outcome of H. pylori infections varies among individuals depending, in part, on the environmental factors 1. H. pylori has a pathogenic role in the biologic process of peptic ulcus, gastric carcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma[1,2,7-9]. H. pylori expressed vacuolating cytotoxin (vacA) activity and possessed the cagA gene. Both of these pathogenic factors have been associated with an increased incidence of gastric cancer in humans[3]. Correa et al. found that H. pylori infection increased the risk of gastric atrophy ninefold and intestinal metaplasia fourfold, compared with absence of infection[10]. Watanabe et al. published the results of a study about the effect of H. pylori infection on animal model and found 37% of Mongolian gerbils had developed gastric adenocarcinoma at 62 weeks. These cancers, as in human, were located distally and developed on a background of intestinal metaplasia[11]. Guslandi expressed the possible mechanism of gastric pathology and H. pylori infection implies, throughout the inflammatory infiltration, a series of alterations, which are the increase of reactive oxygen species, the reduction of antioxidant substances, the alteration of the balance between pro-inflammatory and anti-inflammatory cytokines, and the alteration of the relation cellular hyperproliferation and apoptosis[12]. Uemura et al. found that postsurgical eradication of the bacterium appeared to inhibit development of new cancer[13]. Bayerdorffer et al. presented that the cure of H. pylori infection with full eradication of the bacteria regresses the lymphoma growth and achieves cure of the tumor[14]. Development of a vaccine against H. pylori might yield promising result in decreasing the incidence of gastric adenocarcinoma[15].LSCC is the second most common respiratory cancer after lung cancer. Its incidence is increasing over time in much of the world and this increase is generally accepted to be related to changes in tobacco and alcohol consumption. Evidence from epidemiological studies which supports the involvement of new risk factors in the etiology of LSCC, as well as new perspectives in therapy, must be taken into consideration in order to realize primary and tertiary prevention[16]. Smoking and alcohol consumption have been considered the most important risk factors for LSCC. Other factors may play some independent role in laryngeal carcinogenesis such as diet. Exposure to asbestos, diesel fumes, mustard gas, and sulphuric acid have been implicated as risk factors for LSCC. Air pollution was found to be a strongly associated with occurrence of LSCC[16].
Evidence from epidemiological studies that supports the involvement of new risk factor in the etiology of LSCC as well as new perspectives in therapy. Grandis et al. reported that no correlation between H. pylori infection and head and neck cancer. They explained this by following reasons:
1- Small sample size
2- Collection of sera in case only at the time of diagnosis
3- An imperfect serologic assay[5].
We previously found that patients with carcinoma of the larynx were different than matched controls to have immunologic evidence of infection with H. pylori[6]. This result encourages us and we examined interference between LSCC and serology of H. pylori and active H. pylori infection in the larynx specimen. We previously reported that seropositivity rate was 73.07 (control was 40.62%) and we found seropositivity rate was 60% in this study. We determined the presence of bacteria 40% of the larynx specimens.
Rezaii et al have found that H. pylori seropositivity is 94.3% in laryngeal carcinoma and 92.6% in hypopharyngeal cancers[17]. In another study it has been found that H. pylori frequency in Turkey varies between 60% and %84 in normal population[3]. When interpreted this way, it does not seem significant that seropositivity is 60% in patients diagnosed with laryngeal carcinoma. However when interpreted histopathologically, presence of H. pylori was detected in 12 (40%) of the specimens from tumoral tissues while it was detected in 6 (20%) only of the specimens from non-tumoral tissues. Additionally, no presence of H. pylori detected in the non-tumoral tissue specimens of patients in whose tumoral tissue specimens H. pylori was not detected histopathologically. It is significant that in tumoral tissues, which are the study group of H. pylori, the presence is detected twice as frequent as in non-tumoral tissue specimens (control group) and this suggests that it is a factor in cancer etiopathogenesis. Titiz et al examined the specimens from 21 patients diagnosed with laryngeal carcinoma and detected H. pylori in 17 of these[18]. This study provides supporting evidences for our results. On the other hand, Kızılay et al found a lack of histologic evidence for H. pylori in laryngeal cancer specimens[19].
H. pylori infected mucosa characteristically exhibits signs of acute inflammation manifested by intraepithelial neutrophils, superimposed upon a background of chronic inflammation shown by lymphocyte infiltration. This inflammation process is suggested to predispose an individual to such diseases like peptic ulcus, adenocarcinoma and lymphomas. Lymphoid follicles are a common feature of Helicobacter pylori-associated gastritis. Lymphoid aggregates were present in 28%-100% of patients with H. pylori and provide a useful marker for H. pylori infection[20-22]. We histopatologically determined that lymphoid aggregates are present 83% of H. pylori positive larynx biopsy specimens (10/12).
The mechanism by which H. pylori may facilitate carcinogenesis was not analyzed in this study. However, these bacteria cannot be implicated in carcinogenesis until the mechanism of action is elucidated, that is, until causality is established.
Conclusion
H. pylori has been linked to the development of both benign and malignant diseases of aerodigestive tract, including duodenal and gastric ulcer, hypertrophic gastropathy, gastric adenocarcinoma, and MALT lymphoma. H. pylori infection may be an initiator or a promoter of malignancies. Chronic infection may play an etiologic role in LSCC and our previous study and this study suggest that H. pylori is the offending organism. Although this study does not establish a definite causal relationship between H. pylori and LSCC, it offers an avenue for further work.Reference
1) Pajares JM. Helicobacter pylori infection and gastric cancer in Spain. Hepatogastroenterology 2001;48(42):1556-1559. [ Özet ]
2) Williams MP, Pounder RE. Helicobacter pylori: from the benign to the malignant. Am J Gastroenterol 1999;94(11 Suppl):11-16. [ Özet ]
3) Us D, Hascelik G. Seroprevalence of helicobacter pylori infection in an asymptomatic Turkish population. J Infect 1998;37(2):148-150. [ Özet ]
4) Shimoyama T, Fukuda Y, Sakagami T, et al. Experimental gastric carcinogenesis related to Helicobacter pylori. Hepatogastroenterology 2001;48(42):1531-1536. [ Özet ]
5) Grandis JR, Perez-Perez GI, Yu VL. Lack of serologic evidence for Helicobacter pylori infection in head and neck cancer. Head Neck 1997;19(3):216-218. [ Özet ]
6) Aygenc E, Selcuk A, Celikkanat S, Ozbek C, Ozdem C. The role of Helicobacter pylori infection in the cause of squamous cell carcinoma of the larynx.
Otolaryngol Head Neck Surg 2001;125(5):520-521. [ Özet ]
7) Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 1991;325(16):1132-1136. [ Özet ]
8) Valle J, Gisbert JP. Helicobacter pylori infection and precancerous lesions of the stomach. Hepatogastroenterology 2001;48(42):1548-1551. [ Özet ]
9) Shiotani A, Nurgalieva ZZ, Yamaoka Y, Graham DY. Helicobacter pylori. Med Clin North Am 2000;84(5):1125-1136. [ Özet ]
10) Correa P. Helicobacter pylori and gastric cancer. Am J Surg Pathol 1995;19(Suppl):37-43. [ Özet ]
11) Watanabe T, Tada M, Nagai H, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 1998;115(3):642-648. [ Özet ]
12) Guslandi M. A radical view of Helicobacter pylori. Am J Gastroenterol 1999; 94: 2797-2798. [ Özet ]
13) Uemura N, Okamoto S. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer in Japan. Gastroenterol Clin North Am 2000;29(4):819-827. [ Özet ]
14) Bayerdorffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995;345(8965):1591-1594. [ Özet ]
15) Peterson WL.Helicobacter pylori and gastric adenocarcinoma. Aliment Pharmacol Ther 2002;16(Supp 1):40-46. [ Özet ]
16) Cattaruzza MS, Maisonneuve P, Boyle P. Epidemiology of laryngeal cancer. Eur J Cancer B Oral Oncol 1996;32B:293-305. [ Özet ]
17) Rezaii J, Tavakoli H, Esfandiari K, Ashegh H, Hasibi M, Ghanei G et al. Association between Helicobacter pylori infection and laryngohypopharyngeal carcinoma: a case–control study and review of the literature. Head & Neck2008 December,1624-27 [ Özet ]
18) Titiz A, Ozcakir O, Ceyhan S, Yilmaz YF, Unal A , Akyon Y The presence of Helicobacter pylori in the larynx pathologies Auris Nasus Larynx 35 (2008) 534–538 [ Özet ]
19) Kızılay A, Saydam L, Aydin A, Kalcioglu M.T , Özturan O Aydin NE Histopathologic examination for Helicobacter pylori as a possible etiopathogenic factor in laryngeal carcinoma. Chemotherapy 2006;52:80–82 [ Özet ]
20) Burstein M, Monge E, Leon-Barua R, et al. Low peptic ulcer and high gastric cancer prevalence in a developing country with a high prevalence of infection by Helicobacter pylori.J Clin Gastroenterol 1991 Apr;13(2):154-156 [ Özet ]
21) Genta RM, Hamner HW. The significance of lymphoid follicles in the interpretation of gastric biopsy specimens. Arch Pathol Lab Med 1994 Jul;118(7):740-743 [ Özet ]
22) Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991 Nov 9;338(8776):1175-1176. [ Özet ]




