THE PROTECTIVE ROLE OF TRIMETAZIDINE ON OXIDATIVE SYSTEM AND TEOAEs IN RABBITS EXPOSED TO NOISE
2Department of Biochemistry, University of Kocatepe, Afyon, Turkey
Summary
Objective: Trimetazidine (TMZ) is an antianginal drug, which has potent antioxidant activity on various tissue. The aims of this study were to uncover the effects of noise exposure on oxidative status and hearing thresholds and to investigate possible protective role of drug TMZ on oxidative system and hearing thresholds.Materials and Methods: Two groups of rabbits were used in this study and each group had eight rabbits. Eight rabbits in the first group (control group) were not given any treatment whereas TMZ (20 mg/kg p.o, tid, 7 days) was given to the other eight rabbits in the second group. TEOAEs were recorded in all animals before and after noise exposure. As oxidative stress parameters plasma MDA levels as well erythrocyte GSH, GP and GR enzyme activities were measured in all rabbits. All the rabbits were exposured to noise (100 dB SPL, 1000 Hz, 1 h) and then TEOAEs were recorded again.
Results: In the first group, after noise exposure plasma MDA levels were found to be increased (p<0,05) whereas erythrocyte GSH, erythrocyte GP, erythrocyte GR levels were found to be decreased ( p<0,05). In the second group which was given trimetazidine plasma MDA levels were found to be increased (p<0,05) but erythrocyte GSH, erythrocyte GP, erythrocyte GR levels did not changed ( p>0,05) . TEOAEs after noise exposure were weaker in both groups, but reproducibility and signal noise ratio levels were higher in the second group (p<0.05).
Conclusions: It was shown in this study that cochlear damage due to noise exposure detected by OAE changes was prevented by TMZ in rabbits and this effect of TMZ is possibly mediated via its antioxidant properties. In our opinion, this drug which is used for treatment of ischemic heart diseases and episodic vertigo can play a role in prevention of noise induced hearing loss in people working in hazardous are as.
Introduction
Trimetazidine (TMZ) is an antianginal drug, which has potent antioxidant activity on various tissue [1]. It has been used as an active drug in ischemic heart diseases, hepatic ischemia and nephrotoxicity induced by cyclosporine [2,3,4] Clinical studies have shown that TMZ also reduces duration and frequency of vertigo, improves cochleo-vestibular disorders associated with Meniere's disease (tinnitus, deafness and vertigo) and with ischemia. It was also shown to be effective on isolated tinnitus and has beneficial effects on sudden sensorineural hearing loss [5,6,7,8]. The mechanism of action of TMZ has been studied on different tissues. TMZ inhibits the excessive release of oxygen free radicals and reduces the release of inorganic phosphate and efflux of phosphocreatine. TMZ also protects ATP stores, reduces membrane lipid peroxidation and induces preferential utilization of exogenous glucose [1]. Additionally, TMZ preserves the physiological activity of isolated cardiac cells submitted to hypoxia [9]. Moreover, TMZ probably acts on the control of the intracellular pH and, consequently, modulates ionic fluxes (Na+/H+, Na+/Ca2+, Na+/K+-ATPase, etc.) [10, 11]. TMZ most probably prevents intracellular swelling by inhibition of an excessive intracellular Na+/Ca2+ store, and preventing the loss of intracellular K+ [12].The precise mechanism leading to tissue damage in noise trauma is not known. Noise-induced hearing loss (NIHL) is a sensorineural deafness which appears classically as a notch at 3,4 or 6 kHz in a pure-tone audiogram [13]. The initial response to hazardous noise is the decreased microcirculation due to vasoconstriction and localized edema with vascular permeability increase [14]. Lipid peroxidation and free radical species (FRS) generated after prolonged hypoxia and ischemia periods were found to be responsible for damages to inner and outer hair cells [15, 16]. The presence malondialdehyde (MDA) which is a byproduct of lipid peroxidation indicates that membrane lipids have been subjected to oxidative damage [17, 18]. Erythrocyte glutathione (GSH), erythrocyte glutathione peroxidase (GP) and erythrocyte glutathione reductase (GR) are mainly intracellular antioxidants protecting the cells against free radical damage [19].
Transient evoked otoacoustic emissions (TEOAEs) test is widely used in the diagnosis of cochlear disease like noise-induced hearing loss [20]. The aims of this study were to uncover the effects of noise exposure on oxidative status and hearing thresholds and to investigate possible protective role of drug trimetazidine on oxidative system and hearing thresholds.
Methods
Sixteen rabbits (New Zealand strain, 1.80-1.95 kg) were used in this study. The experimental protocol was approved by the Animal Ethic Committee at Afyon Kocatepe University. The rabbits were divided into two groups 8 rabbits each. The rabbits in the first group were not given any treatment whereas a total dose of 20 mg/kg/day TMZ was given per orally tid for 7 days to each rabbit in the second group. On the 8th day attempts to record TEOAEs in the animals were made first without anesthesia, i.e. all the rabbits were alert during the whole study (Homoth T-OAE diagnostic system, Hamburg). Left ears were used for hearing tests and right ears were used to obtain blood specimens. During the hearing measurements, a 24-mm probe was inserted deeply into the ear canal while the animal was placed in a small wooden box open at the top in a sound-proofed booth. After recording the TEOAEs, 2 ml peripheral blood was obtained from each rabbits to measure oxidative parameters. Then all the rabbits were exposed to noise (100 dB SPL, 1000 Hz, Ih) in a sound exposure cage of dimensions 1.5x0.75x0.75 m which were used for generation of narrow band noise (1000 Hz) presenting free-field from loudspeakers (Interacoustics AD 229 audiometer). The sound was calibrated and measured in the cage to ensure uniformity of the stimulus using a sound level meter (Testo 816, Germany). Immediately after noise exposure, the rabbits were removed from the cage and within 30 min TEOAEs were recorded again. Finally within 1 h of exposure, blood samples were taken from the animals for biochemical measurements.Biochemical Methods: Blood samples were drawn into heparinized tubes. After 10 min centrifugation at 2500 rpm and +4°C, plasma and buffy coat were removed. Erythrocytes were washed three times with ice-cold physiologic saline. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Plasma MDA levels were determined using a method of Ohkawa et al. [21]. Erythrocyte GSH levels were determined according to Beutler et al. [22]. Erythrocyte GPx and GR activities were measured with a Hitachi 917 autoanalyzer using commercial kits obtained from Randox Laboratories (Randox Laboratories Ltd. Antrim, UK.).
Data were statistically evaluated by SPSS for windows (SPSS Inc., Chicago, II, USA). Mann-Whitney U test was used for the comparison of the oxidative parameters and TOAEs between two groups. Wilcoxon signed rank test was used for the comparison of pre and postoperative data within each group.
Results
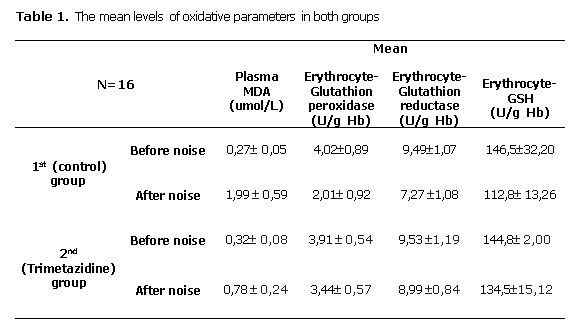
When oxidative parameters before noise exposure were compared, no difference was dedected between 1st (control) and 2nd (trimetazidine) group (p>0,05) (Table 1). In the first group, after noise exposure plasma MDA levels were found to be increased (p<0,05) whereas erythrocyte GSH, erythrocyte GP and erythrocyte GR levels were found to be decreased ( p<0,05) (Table 1). In the second group which was given trimetazidine plasma MDA levels were found to be increased (p<0,05) but no statistically significant change was detected in erythrocyte GSH, erythrocyte GP and erythrocyte GR levels ( p>0,05) (Table 1) .
When oxidative parameters after noise exposure were compared between 1st (control) and 2nd (trimetazidine) group MDA levels were found to be lower (p<0,05) whereas eGSH, eGP eGR levels were found to be higher ( p<0,05) in the second group (Table 1).
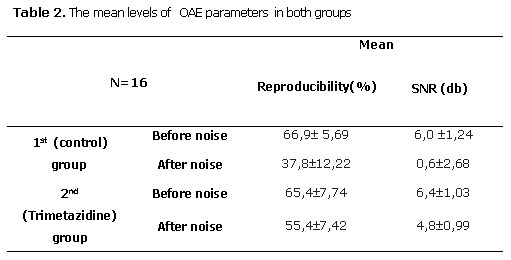
TEOAEs after noise exposure were weaker in both groups (p<0.05), but reproducibility and signal noise ratio levels were significantly higher in the second group (p<0.05) (Table 2).
Discussion
It is shown in the previous studies that acoustic trauma causes free radical formation in inner ear [19, 23, 24, 25]. Noise exposure also causes oxidative stress for all the body and this condition is not limited to cochlear tissue [19]. According to some authors these free radical species are responsible for noise or drug induced hearing loss and antioxidants may play a protective role [19,26 ,27].The destructive effects of oxygen free radicals on cochlea can be attributed to:
1. Impairment of mitochondrial energy production in basolateral membrane of cochlea causing a decrease in rate of endolymph secretion [1]
2. Loss of intracellular K+ due to membrane lipid peroxidation which causes a passive K+ permeability of the cell membrane [1]
3. Impairment of evoked release of neurotransmitters from the hair cells due to modification of synthesis and release of these neurotransmitters. This deleterious action can be ascribed to the ability of oxygen free radicals to induce protein breakdown and reduction of enzymic metabolism [1]
Quirk et al reported [15] that lazaroid, U74389F, a lipid peroxidation inhibitor provides partial protection from noise-induced temporary threshold shifts. The study of Seidman et al. [28] demonstrated that SOG-polyethylene glycol and allopurinol attenuate noise-induced temporary threshold shifts. It was suggested that brief antioxidant loading with pharmacological doses can reduce high energy noise induced oxidative stress [29]. Application of ascorbic acid before the noise exposure resulted in a lower or no loss of hair cells within cochlea [30]. Kaygusuz et al. demonstrated that melatonin prevents increase of MDA and decrease of erytrocyte GP levels thus protects hearing thresholds [31].
In the present study plasma MDA levels were increased significantly in both the control and the TMZ group after noise exposure. But when we compare the post-noise MDA levels. It is obviously seen that MDA levels in TMZ group were significantly lower than the saline group. Additionally, no significant change was detected after noise exposure in GSH, erythrocyte GP and erythrocyte GR levels in TMZ group in contrast to control group. These results show that TMZ avoided consumption of free radical scavenging molecules in plasma.
Lamm and Arnold [32] reported that exposure to broad band noise induces cochlear hypoxia and ischemia and TMZ was shown to prevent free radical formation in ischemic conditions during reoxygenation period in rat heart cells [33]. In light of these data we think that TMZ similarly avoids free radical formation in cochlea like it does in heart cells.
It has recently been shown that TMZ is capable of increasing membrane phospholipid turnover which could account for the cytoprotective effect observed in cardiac cells [34, 35]. This action of TMZ may have some role especially on restoring membrane lipid compositition of cochlear cells after lipid peroxidation avoding passive K+ permeability .
Aubert et al. [1] in their study concluded that TMZ has beneficial action on bioelectrical potential generation when the semicircular canal was exposed to phenazine methosulphate (a substance which is a generator of free radicals) on frog model. According to Gil-Loyzaga et al. [36] TMZ prevents cochlear damage after intraperitoneal and perilymphatic administration of kainic acid.
In this study, after noise exposure the percentage reproducibility and SNR levels as parameters of TEOAEs test showed significant decrease in both of two rabbit groups. Xu et al. [37] have demonstrated that with increasing hearing loss, percentage reproducibility becomes much weaker. However reproducibility and SNR levels in the rabbits treated by TMZ were found to be higher than the control group. This result shows that TMZ attenuates noise-induced hearing threshold shifts and it appears to play a protective role for cochlea to some extent. Regarding our results it can be suggested that, protective role of TMZ is related to its capacity to prevent oxygen free radical formation.
In conclusion, it is shown for the first time that trimetazidine prevents noise induced cochlear damage by avoiding free radical formation in rabbits. In our opinion, TMZ which is used in ENT practice for treatment of episodic vertigo and tinnitus may play a role in prevention of noise induced hearing loss in people working in hazardous areas. For this reason it will be useful to conduct further clinical studies in human subjects testing the efficacy of TMZ to protect against noise induced cochlear damage.
*Presented on the 10th Asia-Ocenia ORL-HNS Congress February 22-26 2004 Kuala Lumpur, Malaysia and won the best poster in Otology award.
Reference
1) Aubert A, Bernard C, Clauser P, Harpey C, Vaudry H. Effect of phenazine methosulfate on electrophysiological activity of the semicircular canal: antioxidant properties of trimetazidine. Eur J Pharmacol 1989;174:215-25. [ Özet ]
2) Tsimoyiannis EC, Moutesidou KJ, Moschos CM, Karayianni M, Karkabounas S, Kotoulas OB. Trimetazidine for prevention of hepatic injury induced by ischaemia and reperfusion in rats. Eur J Surg 1993;159:89-93. [ Özet ]
3) Salducci MD, Chauvet-Monges AM, Tillement JP, Albengres E, Testa B, Carrupt P, Crevat A. Trimetazidine reverses calcium accumulation and impairment of phosphorylation induced by cyclosporine A in isolated rat liver mitochondria. J Pharmacol Exp Ther 1996;277:417-22. [ Özet ]
4) Creagh T, Lanigan D, Dolan J, Tormey W, Walshe JJ, Bouchier-Hayes D. Pharmacological manipulation of acute cyclosporin ischemic renal injury with trimetazidine. J Urol 1993;149:915-7. [ Özet ]
5) Kluyskens P, Lambert P, D\\\'Hooge D. [Trimetazidine versus betahistine in vestibular vertigo. A double blind study] Ann Otolaryngol Chir Cervicofac 1990;107 Suppl 1:11-9. [ Özet ]
6) Martini A, De Domenico F. Trimetazidine versus betahistine in Meniere\\\'s disease. A double blind method. Ann Otolaryngol Chir Cervicofac 1990;107 Suppl 1:20-7 (Abstract). [ Özet ]
7) Wayoff M. Evaluation of the therapeutic efficacy of vastarel 20 mg (trimetazidine) in cochleovestibular syndromes. A double-blind study versus placebo. Ann Otolaryngol Chir Cervicofac 1984; 101:565-9 (Abstract). [ Özet ]
8) Beutter P, Guinard F, Jalbert D, Marsac A, Morin R, Sauvage JP, Soudant J. Value of the administration of trimetazidine associated with hemodilution in the treatment of sudden deafness. Report of a multicenter study. Ann Otolaryngol Chir Cervicofac 1990;107:345-50 (Abstract). [ Özet ]
9) Fantini E, Athias P, Demaison L, Grynberg A. Protective effects of trimetazidine on hypoxic cardiac myocytes from the rat. Fundam Clin Pharmacol 1997;11:427-39. [ Özet ]
10) Lagadic-Gossmann D, Le Prigent K, Feuvray D. Effects of trimetazidine on pHi regulation in the rat isolated ventricular myocyte. Br J Pharmacol 1996; 117:831-8. [ Özet ]
11) Renaud JF. Internal pH, Na+, and Ca2+ regulation by trimetazidine during cardiac cell acidosis.Cardiovasc Drugs Ther 1988; 1:677-86. [ Özet ]
12) Labrid C. Cellular disorders induced by ischemia. The effect of trimetazidine Presse Med 1986; 15:1754-7 (Abstract). [ Özet ]
13) Alberti PW Noise and the ear. In: Kerr AG, Stephens D (eds) Scott-Brown\\\'s Otolaryngology, Oxford: Butterworth-Heinemann, 1997; 2/11/8.
14) Axelsson A, Vertes D, Miller J. Immediate noise effects on cochlear vasculature in the guinea pig. Acta Otolaryngol 1981;91:237-46. [ Özet ]
15) Quirk WS, Shivapuja BG, Schwimmer CL, Seidman MD. Lipid peroxidation inhibitor attenuates noise-induced temporary threshold shifts. Hear Res 1994 ;74:217-20. [ Özet ]
16) Oestreicher E, Arnold W, Ehrenberger K, Felix D. New approaches for inner ear therapy with glutamate antagonists. Acta Otolaryngol 1999;119:174-78. [ Özet ]
17) Jackson P, Loughrey CM, Lightbody JH, McNamee PT, Young IS. Effect of hemodialysis on total antioxidant capacity and serum anioxidants in patients with chronic renal failure. Clin Chem 1995; 41:1135-38. [ Özet ]
18) Chapman ML, Rubin BR, Gracy RW. Increased carbonyl content of proteins in synovial fluid from patients with rheumatoid arthritis. J Rheumatol 1989;16:15-9. [ Özet ]
19) Dereköy FS, Dündar Y, Aslan R, Çangal A. Influence of noise exposure on antioxidant system and TEOAEs in rabbits. Eur Arch Otorhinolaryngol 2001;258:518-22. [ Özet ]
20) Kvaerner KJ, Engdahl B, Arnesen AR. Temporary threshold shift and otoacoustic emissions after industrial noise exposure. Scan Audiol 1995; 24:137-41. [ Özet ]
21) Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidase in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-8. [ Özet ]
22) Beutler E, Robson MJ, Buttenwieser E. The glutathione instability of drug sensititive red cells. J. Lab Clin Med 1957; 49:84.
23) Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngology 1995;252:504-8. [ Özet ]
24) Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol 1999;4:229-36. [ Özet ]
25) Kaygusuz I, Ozturk A, Ustundag B, Yalcin S. Role of free oxygen radicals in noise-related hearing impairment. Hear Res 2001;162:43-7. [ Özet ]
26) Henderson D, McFadden SL, Liu CC, Hight N, Zheng XY. The role of antioxidants in protection from impulse noise. AnnN Y Acad Sci 1999; 28:368-80. PMID: 10842607
27) Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res 1998; 784:82-90. [ Özet ]
28) Seidman MD, Shivapuja BG. The protective effects of allopurinol and superoxide dismutase on noise-induced cochlear damage. Otolaryngol Head Neck Surg 1993; 109:1052-6. [ Özet ]
29) Elsayed MM, Armstrong KL, William MT, Cooper MF. Antioxidant loading reduces oxidative stress induced by high energy noise (blast). Toxicology 2000;155:91-9. [ Özet ]
30) Branis M, Burda H. Effects of ascorbic acid on the numerical hair cell loss in noise exposed guinea pigs. Hear Res 1988;33:137-40. [ Özet ]
31) Karlidag T, Yalcin S, Ozturk A, Ustundag B, Gok U, Kaygusuz I, Susaman N. The role of free oxygen radicals in noise induced hearing loss: effects of melatonin and methylprednisolone. Auris Nasus Larynx 2002;29:147-52. [ Özet ]
32) Lamm K, Arnold W. Noise-induced cochlear hypoxia is intensity dependent, correlates with hearing loss and precedes reduction of cochlear blood flow.. Audiol Neurootol. 1996; 1:148-60. [ Özet ]
33) Lavanchy N, Martin J, Rossi A. Anti-ischemic effects of trimetazidine: 31P-NMR spectroscopy in the isolated rat heart. Arch Int Pharmacodyn Ther 1987;286:97-110. [ Özet ]
34) Sentex E, Sergiel JP, Lucien A, Grynberg A. Trimetazidine increases phospholipid turnover in ventricular myocyte. Mol Cell Biochem 1997;175:153-62. [ Özet ]
35) Sentex E, Sergiel JP, Lucien A, Grynberg A. Is the cytoprotective effect of trimetazidine associated with lipid metabolism? Am J Cardiol 1998; 82:18K-24K. [ Özet ]
36) Gil-Loyzaga P, Hernandez E, Carricondo F, Simon F, Poch-Broto J. Trimetazidine prevents cochlear lesions induced by intraperitoneal and perilymphatic administration of kainic acid. Brain Res 1999;826:95-103. [ Özet ]
37) Xu ZM, Vinck B, De Vel E, Van Cauwenberge PB. Mechanisms in noise-induced permanent hearing loss: an evoked otoacoustic emission and auditory brainstem response study. J Laryngol Otol 1998;112:1154-61. [ Özet ]