THE EVALUATION OF PAIN RELIEF AFTER MASSETER BOTILINUM TOXIN INJECTION ON BRUXISM
Summary
Bruxism is defined as abnormal maxillomandibular activity, characterized by grinding and clenching of the teeth, which can lead to wear on tooth surface, sensitivity, pain in the teeth, jaw, masticatory muscle and temporomandibular joint (TMJ), headache, ear pain, oral and facial pain, prostheses/implant failure and even tooth loss. Although it is known to be mainly during sleep, but in case of patients" anxiety, it may also occur during daily life. There are various treatment modalities of bruxism such as oral splint, behavioral approaches, medications but none is widely accepted. Botox is widely used and accepted both in cosmetic purposes and also noncosmetic conditions such as cervical dystonia, severe hyperhidrosis, TMJ dysfunction, bruxism and headache. Bruxism related referred otalgia is known to be a common symptom to ENT clinics. The purpose of this study is to investigate the performance of BTX-A injection to masseter muscle on bruxism by comparing the pain scores and frequency before the injection and following 2nd and 4th weeks after BTX-A injection. The reduction of the pain significantly improves the quality of life and botox injection to masseter has found to be quite effective. Although several limiting factors such as high cost and the probable necessity of repeated injections, in cases with insufficient response to conservative treatment methods, botulinum toxin may be an alternative and effective treatment for nocturnal bruxism and TMJ oriented otalgia.Introduction
Bruxism is defined as abnormal maxillomandibular activity, characterized by grinding and clenching of the teeth. Bruxism can lead to wear on tooth surface, sensitivity, pain in the teeth, jaw, masticatory muscle and temporomandibular joint (TMJ), headache, ear pain, oral and facial pain, prostheses/implant failure and even tooth loss[1,2,3]. Bruxism can be associated with the unconscious and intense contractions of masseter and temporalis muscles. Although it is known to be mainly during sleep, but in case of patients" anxiety, it may also occur during daily life[3,4]. There are various treatment modalities of bruxism such as oral splint, behavioral approaches, medications (muscle relaxants, antidepressants, neuropathic agents) but none is widely accepted[5].Botulinum toxin A (BTX-A) is an exotoxin produced by the bacterium Clostridium botulinum, which blocks acetylcholine release from cholinergic nerve endings through the neuromuscular junction, resulting in inactivity of muscles or glands[3-5]. Botox is widely used and accepted both in cosmetic purposes and also noncosmetic conditions such as cervical dystonia, severe hyperhidrosis, sialorrhea, strabismus, blepharospasm, hemifacial spasm, TMJ dysfunction, bruxism, masticatory myalgias and headache. In case of bruxism, BTX-A injections are directly applied into the masseter and/or temporalis muscles to relax these muscles and reduce the clenching power[1,4,6]. The clinical effects are typically seen on the first week after the injection, followed by 3 to 8 weeks maximum effect and the typical duration of the effect is three to four months[6-9].
The purpose of this study is to investigate the performance of BTX-A injection to masseter muscle on bruxism by comparing the pain scores and frequency before the injection and following 2nd and 4th weeks after BTX-A injection.
Methods
In the period between March 2021- December 2021, prospective analyses made of the data of 102 patients (72 female and 30 male) who underwent BTX-A injections to masseter muscle for clinically diagnosed as bruxism. Exclusion criteria were TMJ disorders (subluxation, dislocation and ankylosis), ongoing psychological or neuropathy therapy, pregnancy and lactation. Also, on the basis of the patients" volunteering; an informed consent was taken to be a participant of this research. The informed consent had detailed information about both the botulinum toxin injection and the research protocol.The research protocol was also approved by the instutional review board of the hospital.
For evaluation of the pain, Visual Analogue Scale (VAS) forms were completed by the patients scoring the degree of the pain between 0 (absence of pain) and 10 (maximum pain) before the injection and then at 2nd and 4th week after the injection. Patients were also interrogated about the frequency of the pain due to bruxism as day/fortnight with a frequency chart on information form.
The following data were recorded for all patients; age, sex, duration of the complaint before the injection, the time that the first effect was seen, the time when effectiveness started to be lost and also there was any complaint after BTX-A injection such as difficulty in speaking, chewing, smiling.
In all procedures; 100 MU BTX-A (Botox, Allergan, Inc., Irvine, CA, USA) was diluted with 2ml of saline solution. For all cases, a dose of 24 MU of BTX-A was injected into a single masseter muscle using 0.5 mL insulin syringe at three points (8 MU/injection).
The patient was requested to clench the masseter muscle and a "safety zone" was detected as the anterior and posterior borderline of the masseter, the mandibular line and an imaginary line lying from the ear lobe to the oral commissure (Figure 1). BTX-A injections were performed in the safety zone.
 Büyütmek İçin Tıklayın |
Figure 1: Safety zone for the masseter BTX injection; The patient was requested to clench the masseter muscle and a "safety zone" was detected as the anterior and posterior borderline of the masseter, the mandibular line and an imaginary line lying from the ear lobe to the oral commissure. |
Statistical analysis was performed with the SPSS (version 20.0, Chicago, USA). The changes of the VAS scores were tested with the Friedman test to analyze the variations of the pain scores. The value of p < 0.05 was considered statistically significant. The changes in the frequency of the pain results were tested with the Friedman test and a value of p < 0.05 was considered as statistically significant. The binary comparisons of the data were divided into 2 groups as before the injection-the 2nd week results comparison, the 2nd week results-4th week results comparison. Each group was analyzed with the Durbin-Conover test and the value of p < 0.05 was considered as statistically significant.
Results
Evaluation was made of the data of 102 patients with a mean age of 36,5 ± 7,3 years (ranging between 23-55 years). The mean duration of complaint before injection was 5 (1 - 25) years. General information of the cases was summarized on Table 1.Table 1: General descriptive information of the cases
Five patients had pain on the injection points which had lasted for 1-3 days. 2 cases had unilateral marginal mandibular nerve paresis lasted for 3-4 weeks. None of the cases had reported dry mouth. There was no statistically significant change in respect of the maximum mouth opening. Nine of the cases had not like the reshapening of the face; less prominent jawline, longer and thinner cheeks (Figure 2).
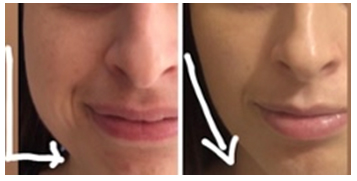 Büyütmek İçin Tıklayın |
Figure 2: Reshapening of the jawline 4 week after masseter muscle BTX injection. |
The time that effects were first seen was 10.24 ± 2.02 days, and the time that the loss of effectiveness started was 4.16 ± 1.21 months. Nineteen of the cases (12 female and 7 male) needed additional BTX-A injection to unilateral masseter muscle because of no significant improvement in the pain scores at the 2nd week. These cases declared pain relief on the 4th week control.
The differences between before injection and post-injection (2nd week-4th week) pain values were analyzed with Friedman test and statistically significant changes were found p value 0.001 (Table 2).
Table 2: The differences of the pain score and pain frequency, before and after BTX-A injection
Bruxism related headache frequency changes were also recorded, analyzed, tested with the Friedman test and the results were found to be statistically significant p value <0.001. The binary comparisons of the data divided into two groups as before the injection-the 2nd week results, the 2nd week-4th week results. Each group was analyzed with the Durbin-Conover test and statistically significant changes were found p value <0.001 (Table 3).
Table 3: Binary comparisons of the pain score and pain frequency
Discussion
The first usage of BTX-A in the treatment of bruxism was a young female patient with a brain injury and a significant reduction in clenching power was observed during the effectiveness period of botox[1]. Since then, botulinum toxin-A has been used for the treatment of bruxism for different pathologies such as cranial/cervical dystonia, Huntington"s disease and autism[1,4,6,8,9].Both BTX-A and BTX-B serotypes of botulinum toxin have been approved by the US Food and Drug Administration for clinical usage in the treatment of bruxism; Lang reported that BTX- A showed better pain relief than BTX-B[10]. In this research, BTX-A was preferred for masseteric injections in the treatment of bruxism.
Although the pathogenesis of bruxism remains unclear, it is accepted as multifactorial etiology in nature. It may occur as a result of possible physical (malocclusion, sleep apnea) or psychological conditions (emotional stress, anxiety, aggressive or hyperactive personality types). Therefore; the treatment of bruxism could be difficult for both clinicians and patients, because most of the cases involve multiple components such as somatic and psychogenic components[11,12], Bruxism might lead to pain in the head, neck, jaw, teeth and TMJ. However, conservative treatments may be limited in the resolution of this pain problem. In the past few years, the usage of botulinum toxin therapy has become one of the promising sources of bruxism management. It was reported that BTX-A injection reduced the number of sleep bruxism events[10-11]. Similarly, Santamato et.al reported that neck pain related to nocturnal bruxism can be treated with BTX-A injection; 40 MU to each masseter and 25 MU to each temporalis muscle[13].
In a randomized, double blind, placebo-controlled trial, Shim et.al reported that a single BTX-A injection decreased the masseter muscle contraction intensity in sleep bruxism with polysomnographic evaluation for 12 weeks[14]. The decrease in the muscle contraction power and intensity reduced the abnormal clenching activity, bruxism, and this was found to be similar and compatible with our analyzes.
It was also reported in the literature that the maximum mouth opening may be increased after BTX-A injection to the masseter muscle. Some researchers showed that the interincisal distance could be induced with BTX-A injection[15,16,17]. Altaweel et.al compared intraoral BTX-A injection with extraoral BTX-A injection to the lateral pterygoid muscle in patients with TMJ anterior disc replacement with reduction. A significant improvement in mouth opening was recorded with both aforementioned approaches[18]. However; the results of our study was not analyzed through that path due to none of the patients having previous complaint of limited mouth opening.
Temporomandibular joint injuries such as subluxation, dislocation and ankylosis were exclusion criteria for this research. Fu et.al. injected BTX-A to the lateral pterygoid muscle in 5 cases with habitual TMJ dislocation and became successful; the dislocation was cleared up from 3 months to 2 years[19]. In a different research by Robiony, it was determined that abnormal clenching activity was disappeared with a single dose of BTX-A injection, further repeated injections was not required[17]. Another research by Renapurkar et.al compared injectable agents versus surgery in patients with recurrent TMJ dislocation and showed that lateral pterygoid muscle BTX-A injection should be done to prevent dislocation; neither masseter muscle BTX-A injection nor temporalis muscle BTX-A injection could prevent dislocation[20]. The authors claimed that minimally invasive injectable agents have shown promising results, with low risk, compared with the surgical techniques hence they should be the initial treatment for recurrent mandibular dislocation[17,19,20].
Another controversy about masseter muscle BTX-A injection is the volume loss of mandible or osteopenia[2,4,21]. There is not enough data as clinical research in case of mandibular osteopenia (review). Lee et.al claimed that repeated injections of BTX-A have caused bone loss on the mandibular angle, whereas Chang et.al found out there was not statistically significant change in bone volume.
The efficacy of BTX-A injection versus dry or wet needle application for pain control in bruxism patients, was studied and compared in various studies. Nowak et.al studied on these researches in a systematic review and put forward that dry or wet needle application techniques were more successful on temporalis muscle. However, in case of masseter dysfunction related pain and bruxism, statistically significant difference was not found between the results of aforementioned techniques[16].
The mandibular osteopenia was also claimed as another adverse effect that related with high dose or repeated usage of masseter BTX-A injections. Canales et.al reported a significant decrease in bone volume of coronoid and condylar processes that correlated with the dose of BTX-A. In medium dose group, the bone loss of coronoid process was observed alone; meanwhile in high dose group, both coronoid and condylar processes were affected. However, in low dose group, BTX-A related bone loss was not detected and low dose usage was also recommended by the authors[22]. Raphael et.al also studied on temporomandibular complex" bone volume changes as a result of BTX-A injections and found out that there was not statistically significant difference in bone loss due to multiple BTX-A injections. Nevertheless, it was also stated that in case of high dose (>100mU) injections and multiple usage, the clinicians should be alerted about muscle and bone loss of temporomandibular complex[23].
In conclusion, Botox therapy seems promising and beneficial in the treatment of bruxism, although several limiting factors such as high cost and the probable necessity of repeated injections. In cases with insufficient response to conservative treatment methods, botulinum toxin may be an alternative and effective treatment for nocturnal bruxism and masticatory pain. Future studies with a larger number of patients are required to confirm the conclusions that reached in this study.
Reference
1) Monroy PG, da Fonseca MA. The use of botulinum toxin-a in the treatment of severe bruxism in a patient with autism: a case report. Spec Care Dentist. 2006 Jan-Feb;26(1):37-9. doi: 10.1111/j.1754-4505.2006.tb01508.x. [ Özet ]
2) Beddis H, Pemberton M, Davies S. Sleep bruxism: an overview for clinicians. Br Dent J. 2018 Sep 28;225(6):497-501. doi: 10.1038/sj.bdj.2018.757. Epub 2018 Sep 21. [ Özet ]
3) Nayyar P, Kumar P, Nayyar PV, Singh A. BOTOX: Broadening the Horizon of Dentistry. J Clin Diagn Res. 2014 Dec;8(12):ZE25-9. doi: 10.7860/JCDR/2014/11624.5341. Epub 2014 Dec 5. [ Özet ]; PMCID: PMC4316364.
4) Pihut M, Ferendiuk E, Szewczyk M, Kasprzyk K, Wieckiewicz M. The efficiency of botulinum toxin type A for the treatment of masseter muscle pain in patients with temporomandibular joint dysfunction and tension-type headache. J Headache Pain. 2016;17:29. doi: 10.1186/s10194-016-0621-1. Epub 2016 Mar 24. [ Özet ]; PMCID: PMC4807183.
5) Asutay F, Atalay Y, Asutay H, Acar AH. The Evaluation of the Clinical Effects of Botulinum Toxin on Nocturnal Bruxism. Pain Res Manag. 2017;2017:6264146. doi: 10.1155/2017/6264146. Epub 2017 Jul 5. [ Özet ]; PMCID: PMC5516743.
6) Persaud R, Garas G, Silva S, Stamatoglou C, Chatrath P, Patel K. An evidence-based review of botulinum toxin (Botox) applications in non-cosmetic head and neck conditions. JRSM Short Rep. 2013 Feb;4(2):10. doi: 10.1177/2042533312472115. Epub 2013 Feb 12. [ Özet ]; PMCID: PMC3591685.
7) Connelly ST, Myung J, Gupta R, Tartaglia GM, Gizdulich A, Yang J, Silva R. Clinical outcomes of Botox injections for chronic temporomandibular disorders: do we understand how Botox works on muscle, pain, and the brain? Int J Oral Maxillofac Surg. 2017 Mar;46(3):322-327. doi: 10.1016/j.ijom.2016.11.004. Epub 2016 Nov 28. [ Özet ]
8) Finiels PJ, Batifol D. The use of botulinum toxin in the treatment of the consequences of bruxism on cervical spine musculature. Toxicon. 2014 Mar;80:58-63. doi: 10.1016/j.toxicon.2014.01.004. Epub 2014 Jan 22. [ Özet ]
9) Kwon KH, Shin KS, Yeon SH, Kwon DG. Application of botulinum toxin in maxillofacial field: part I. Bruxism and square jaw. Maxillofac Plast Reconstr Surg. 2019 Oct 1;41(1):38. doi: 10.1186/s40902-019-0218-0. [ Özet ]; PMCID: PMC6768934.
10) Lang AM. A preliminary comparison of the efficacy and tolerability of botulinum toxin serotypes A and B in the treatment of myofascial pain syndrome: a retrospective, open-label chart review. Clin Ther. 2003 Aug;25(8):2268-78. doi: 10.1016/s0149-2918(03)80218-7. [ Özet ]
11) Kuc J, Szarejko KD, Sierpinska T. Evaluation of Orofacial and General Pain Location in Patients With Temporomandibular Joint Disorder-Myofascial Pain With Referral. Front Neurol. 2019 May 29;10:546. doi: 10.3389/fneur.2019.00546. [ Özet ]; PMCID: PMC6549135.
12) Kef K. Application of Botulinum Toxin in Patients with Secondary Otalgia Caused by Bruxism. J Pain Res. 2021 Apr 19;14:1051-1059. doi: 10.2147/JPR.S292550. [ Özet ]; PMCID: PMC8064681.
13) Santamato A, Panza F, Di Venere D, Solfrizzi V, Frisardi V, Ranieri M, Fiore P. Effectiveness of botulinum toxin type A treatment of neck pain related to nocturnal bruxism: a case report. J Chiropr Med. 2010 Sep;9(3):132-7. doi: 10.1016/j.jcm.2010.04.004. [ Özet ]; PMCID: PMC3188369.
14) Shim YJ, Lee MK, Kato T, Park HU, Heo K, Kim ST. Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: a polysomnographic evaluation. J Clin Sleep Med. 2014 Mar 15;10(3):291-8. doi: 10.5664/jcsm.3532. [ Özet ]; PMCID: PMC3927435.
15) Sidebottom AJ, Patel AA, Amin J. Botulinum injection for the management of myofascial pain in the masticatory muscles. A prospective outcome study. Br J Oral Maxillofac Surg. 2013 Apr;51(3):199-205. doi: 10.1016/j.bjoms.2012.07.002. Epub 2012 Aug 4. [ Özet ]
16) Nowak Z, Ch?ci?ski M, Nitecka-Buchta A, Bulanda S, Ilczuk-Rypu?a D, Postek-Stefa?ska L, Baron S. Intramuscular Injections and Dry Needling within Masticatory Muscles in Management of Myofascial Pain. Systematic Review of Clinical Trials. Int J Environ Res Public Health. 2021 Sep 10;18(18):9552. doi: 10.3390/ijerph18189552. [ Özet ]; PMCID: PMC8465617.
17) Robiony M. Intramuscular injection of botulinum toxin as an adjunct to total joint replacement in temporomandibular joint ankylosis: preliminary reports. J Oral Maxillofac Surg. 2011 Jan;69(1):280-4. doi: 10.1016/j.joms.2010.05.042. Epub 2010 Nov 4. [ Özet ]
18) Altaweel AA, Elsayed SA, Baiomy AABA, Abdelsadek SE, Hyder AA. Extraoral Versus Intraoral Botulinum Toxin Type A Injection for Management of Temporomandibular Joint Disc Displacement With Reduction. J Craniofac Surg. 2019 Oct;30(7):2149-2153. doi: 10.1097/SCS.0000000000005658. [ Özet ]
19) Fu KY, Chen HM, Sun ZP, Zhang ZK, Ma XC. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010 Jun;48(4):281-4. doi: 10.1016/j.bjoms.2009.07.014. Epub 2009 Aug 7. [ Özet ]
20) Renapurkar SK, Laskin DM. Injectable Agents Versus Surgery for Recurrent Temporomandibular Joint Dislocation. Oral Maxillofac Surg Clin North Am. 2018 Aug;30(3):343-349. doi: 10.1016/j.coms.2018.04.009. Epub 2018 Jun 1. [ Özet ]
21) Buvinic S, Balanta-Melo J, Kupczik K, Vásquez W, Beato C, Toro-Ibacache V. Muscle-Bone Crosstalk in the Masticatory System: From Biomechanical to Molecular Interactions. Front Endocrinol (Lausanne). 2021 Mar 1;11:606947. doi: 10.3389/fendo.2020.606947. [ Özet ]; PMCID: PMC7959242.
22) De la Torre Canales G, Alvarez-Pinzon N, Muñoz-Lora VRM, Vieira Peroni L, Farias Gomes A, Sánchez-Ayala A, Haiter-Neto F, Manfredini D, Rizzatti-Barbosa CM. Efficacy and Safety of Botulinum Toxin Type A on Persistent Myofascial Pain: A Randomized Clinical Trial. Toxins (Basel). 2020 Jun 15;12(6):395. doi: 10.3390/toxins12060395. [ Özet ]; PMCID: PMC7354430.
23) Raphael KG, Janal MN, Tadinada A, Santiago V, Sirois DA, Lurie AG. Effect of multiple injections of botulinum toxin into painful masticatory muscles on bone density in the temporomandibular complex. J Oral Rehabil. 2020 Nov;47(11):1319-1329. doi: 10.1111/joor.13087. Epub 2020 Sep 19. [ Özet ]; PMCID: PMC7693250.




