EVALUATION OF HEARING LOSS IN PATIENTS WITH OSAS AND THE RELATIONSHIP BETWEEN S100B AND NSE
2Hatay Mustafa Kemal University, Audiology Department, Hatay, Turkey
3Hatay Mustafa Kemal University, Department of Thoracic Medicine, Hatay, Turkey
4Hatay Mustafa Kemal University, Department of Biochemistry, Hatay, Turkey
5Osmaniye Sevgi Hospital, Thoracic Medicine, Osmaniye, Turkey
6Hatay Mustafa Kemal University, Department of Neurology, Hatay, Turkey
Summary
Objective: The aim of the current study was to determine the relationship between serum S100B levels, NSE levels and hearing function in patients with OSAS.Material and Methods: Thirty-five subjects and 30 controls were included in the study. The study group included 35 patients with moderate and severe apnoea (AHI > 15) who were diagnosed by polysomnography (PSG). Control subjects were selected with ESS (Epworth Sleepiness Scale). Serum S100B and NSE levels in the study and control groups were analyzed by ELISA (enzyme-linked immunosorbent assay) and high-frequency audiometry was done for all subjects.
Results: Mean S100B levels and NSE levels of the OSAS group were significantly higher than that of the controls (p = 0.039; p = 0.002, respectively). The hearing thresholds between 125-1,000 Hz and between 4,000-12,000 Hz in the study group were found to be significantly higher than the control group.
Conclusion: We suggest that hearing function should be evaluated in OSAS patients and a professional consultation should be sought for OSAS treatment. In addition, serum S100B and NSE measurements may be valued as biochemical indicators in determining hearing loss risk caused by OSAS.
Introduction
Obstructive sleep apnoea syndrome (OSAS) affects 24% of men and 9% of women aged 30-60 years of age. The prevalence of OSA with associated excessive daytime somnolence is approximately 3% to 7% in adult men and 2% to 5% in adult women. This is a condition characterised by intermittent obstruction of the upper respiratory tract that results in recurrent apnoea or hypopnoea attacks, desaturations and changed sleep patterns [1]. Upper respiratory obstruction in OSAS occurs due to pharyngeal collapse caused by small lumen or increased extraluminal pressure. As a result of this collapse, in OSAS some pathophysiological mechanisms such as nocturnal spaced hypoxia, apnoea, inflammation and oxidative stress occur [2]. OSAS can cause various systematic diseases from simple snoring to serious pulmonary, cardiovascular, endocrine and psychological disorders. Patients with OSAS have daytime sleepiness, morning headaches, decreased memory and concentration capacity, impaired cognitive functions and difficulty in performing daily activities [3,4,5].In recent years there has been a gradual increase in interest regarding analysis of the S100B protein and neuron-specific enolase (NSE) as neurobiological markers for various central nervous system diseases [6]. S100B is a calcium-binding protein dominantly found in the cytoplasm of astrocytes and reaching extracellular space by secretion or because of intracellular lesion; it exists in cerebrospinal fluid and serum and can be measured by immunoassay kits [6,7]. S100B is used as a parameter of astrocyte activation and/or death in various cases of brain damage. Brain disorders associated with peripheric increases of S100B include traumatic brain damage, brain ischemia, neurodegenerative diseases and psychiatric disorders [8]. While an increase in NSE levels shows neuronal damage, an increase in S100B may reflect glial damage or reactive astrogliosis, which is an astrocytic reaction to neuronal damage that may have neuroprotective features [9].
It has been shown in many studies that serum levels of neurobiological markers such as S100B and NSE increased as a result of ischemia caused by hypoxia in OSAS patients. [7,9]. Again, various studies have shown that hypoxia induced by apnea / hypopnea episodes in OSAS patients could cause impaired hearing function [10,11].
In this study, we aimed to investigate the relationship between hearing loss, which may occur due to decreased oxygen support as a result of hypoxia in the cochlea of the patients with OSAS, and serum S100B and NSE levels, which are indicators of ischemic damage due to hypoxia in OSAS patients.
Methods
SubjectsThis study was conducted in Hatay Mustafa Kemal University Medical Faculty Hospital. Sixty-five subjects were included in the study. The study group included 35 patients with moderate and severe apnoea (AHI > 15) who were diagnosed by polysomnography (PSG). The study group consisted of 29 male and 6 female patients. Their main complaints were snoring, witnessed apnoea and daytime sleepiness. Meanwhile, the control group consisted of 30 healthy individuals who were evaluated using the Epworth Sleepiness Scale (ESS). The control group consisted of 20 male and 10 female healthy individuals. Detailed physical examinations of the ear, nose and throat were conducted for all subjects included in the current study. Fasting blood samples were also obtained, and a high-frequency audiometry was done for all subjects.
Subjects with a history of ear disease, conductive hearing loss, acoustic trauma, ototoxic drug use, neurological diseases, cerebellopontine angle tumours, major liver diseases, diabetes mellitus, hypercholesterolemia, chronic kidney diseases, cancer, radiotherapy to the head and neck region, and heavy smokers or alcohol and drug addicts were excluded from the study. Approval for the current study was granted by the Local Ethics Committee (2014/137). Informed consent was obtained from all patients.
Procedures
Polysomnography
The patients were hospitalised in the sleep laboratory overnight and a 55-channel (Alice 5, Philips Respironics, Sleepware G3, Minnesota, USA) device was used for polysomnographic evaluation. During polysomnography, the following were recording during sleep: electrocardiogram (EKG); electroencephalogram (EEG); electromyogram (EMG, submental and tibialis anterior muscle); electrooculogram (EOG); airflow (measured with oronasal thermistor); oxygen saturation (measured by fingertip pulse oximetry); thoracic and abdominal breathing movements; snoring (using a tracheal microphone); and various records such as body positions. All data were recorded and evaluated by the same person. Scoring was performed according to the criteria of the American Academy of Sleep Medicine (AASM) [12].
Airflow arrest for at least 10 seconds was evaluated as apnoea. During sleep, a reduction in air flow of at least 50% and 3% desaturation were considered as hypopnoea. Total apnoea and hypopnoea counts per hour were recorded using the apnoea-hypopnoea index (AHI). The oxygen desaturation index was calculated by dividing the desaturation score by the estimated sleep time. The mean oxygen saturation (SaO2) and minimum SaO2 values recorded during sleep and the desaturation index were considered to be indicative of nocturnal hypoxaemia. Patients with an AHI score of 5 and above were evaluated as having obstructive sleep apnoea syndrome. Patients were divided according to their AHI scores into OSAS neg¬ative (simple snoring; AHI < 5 times per hour); mild (AHI: 5-15); moderate (AHI: 15-30); and severe OSAS (AHI > 30) [13].
Sample collection and biochemical assays
Fasting blood samples were collected by venipuncture from study and control subjects. All samples were centrifuged at 1500 g for 10 minutes at 4°C. Serum samples were aliquoted and stored at -80°C until the analysis. Serum NSE and S100B levels were assayed by the enzyme-linked immunosorbent assay (ELISA) method (Thermo Scientific Multiskan GO, Vantaa, Finland) using commercially available ELISA kits (Diametra, Spello-Perugia, Italy). The assay ranges were 0.625-40 ng/ml for the NSE kit and 0.156-10 ng/ml for the S100B kit. The intra- and inter-assay coefficients of variance (CV; %) for both parameters were 10% and 12%, respectively.
Audiological assessment
The pure tone hearing thresholds of all participants (between 125 and 14,000 Hz) were determined using the Grason Stadler GSI-61 (Grason Stadler, Minnesota, USA) device. Thresholds of 20 dB or better obtained between 500 and 4,000 Hz were accepted as normal hearing [14].
Statistical analysis
The data were analysed using the Statistical Package for Social Sciences version 20.0 for Windows program (SPSS Inc., Chicago, IL, USA). The results of the data analysis were presented as descriptive statistics for continuous data and as mean and standard deviation values for normally distributed parameters. The chi-square test was used to compare the categorical data. For continuous data, Student's t-test and Mann-Whitney U test were used to compare the 2 groups. The relationship between the continuous variables was examined by Pearson correlation analysis. The p value of < 0.05 was considered statistically significant for all analyses.
Results
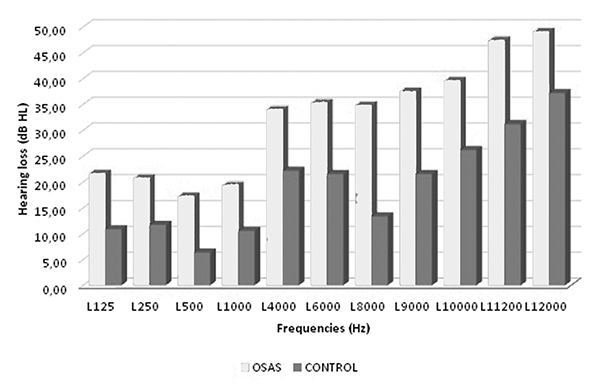
Demographic and clinical features of both groups are given in Table I. Age, sex and body mass index (BMI) were not significantly different between the groups. The mean ESS scores of the study group was significantly higher than that of the control group (p = 0.001). Mean S100B levels and NSE levels of the OSAS group were significantly higher than that of the controls (p = 0.039; p = 0.002, respectively). The mean hearing test results in the study groups vs. the control groups are presented in Table II. There were significant differences in the mean values of the hearing test. The hearing thresholds between 125-1000 Hz and between 4000-12000 Hz in the study group were found to be significantly higher than the control group (Figs. 1 and 2). There was no statistically significant difference in other thresholds including R2000Hz, L2000Hz, R12500Hz, L12500Hz, R14000Hz and L14000Hz. There was no correlation between AHI scores and serum S100B or NSE levels in the OSAS group. In addition, there was no correlation between serum S100B, NSE levels and all frequencies in the hearing test. There was no significant correlation between S100B, NSE and minimum SaO2, mean SaO2, desaturation time or ESS.Table 1: Demographic and clinical features of the both groups
Table 2: Hearing test findings of the both groups
 Büyütmek İçin Tıklayın |
Fig 1: Comparison of OSAS and Control group right ear hearing thresholds |
 Büyütmek İçin Tıklayın |
Fig 2: Comparison of OSAS and Control group left ear hearing thresholds |
Discussion
OSAS is a condition characterised by episodic apnoea/hypopnoea as result of upper-respiratory obstruction during sleep [15]. Apnoea/hypopnoea, causing intermittent hypoxia at the tissue level, occurs secondary to airway obstruction in patients with OSAS. Periodic hypoxia/reoxygenation will cause oxidative stress, inflammation and endothelial dysfunction which results in tissue damage, causing the release of some enzymes and proteins [16,17]. In our study we evaluated the hearing function and serum levels of S100B and NSE in patients with moderate or severe OSAS. We found that patients with OSAS had significantly increased hearing loss and a significant increase in serum levels of S100B and NSE.There are publications in the literature considering the relationship between OSAS and neurobiological markers and there are publications considering the relationship between OSAS and hearing disfunction. To our knowledge this is the first study to address the relationship between hearing loss and neurobiological markers (S100B and NSE) in OSAS patients. S100B and NSE are among the biochemical markers that can be used as an indication of brain damage due to hypoxia [7]. In their study, Da Silva et al. compared S100B values before and after PSG in morbidly obese patients with OSAS and found the S100B values to be significantly higher after PSG. In the same study they could not find a significant difference in NSE values before and after the PSG. They concluded that this is a central nervous system (CNS) astrocytic reaction due to possible cerebral hypoxaemia in morbidly obese patients with OSAS [7]. Jordan et al. researched the biochemical markers S100B and NSE which show brain damage in patients with OSAS and could not find a difference between the patient and control groups [18]. Braga et al. reported that the values of NSE did not change, but they found S100B values to be higher. They indicated in the same study that neither S100B nor NSE showed a significant correlation with AHI score [9]. In our study, no correlation between the degree of OSAS and S100B and NSE values was determined either. Casale et al. indicated in their study that hypoxaemia in OSAS may lead to ischemic damage in the peripheral nerves. They also indicated that, in the first stages of ischemia, various mechanisms activate to reduce peripheric neuropathy; however, they become insufficient over time and, in chronic hypoxaemia, significant neuropathy becomes inevitable [19]. Dziewas et al."s 2007 study showed that recurrent intermittent hypoxaemia can be thought of as a risk factor to peripheric sensory nervous disfunction and, by treating OSAS, recovery in the functions of these nerves occurs [20].
Recently, some studies have been done to determine the effect of OSAS on the central nervous system by measuring more specifically the S100B protein and NSE [9]. Duru et al., in a prospective clinical study including 68 patients diagnosed with OSAS, found the serum S100B concentration to be significantly higher in patients with OSAS compared to the control group [21]. In another study, the serum S100B protein degree in hypoxia following cardiac arrest was found to be correlated to the clinical symptom and coma phase [22,23]. In our study, S100B and NSE values in patients with OSAS were found to be significantly higher compared to the control group (Table I; p < 0.05).
OSAS may cause cerebral vascular insufficiency and ischemic damage in the cochlea as a result of decreased cerebral blood flow during apnoea attacks, hypoxia and acute hemodynamic change [24-28]. Because of the single terminal arterial supply and insufficient collateral circulation, both the cochlea and acoustic nerve are highly sensitive to circulation changes [19,29]. The transmission mechanism of the inner ear depends on the cochlea's oxygen support; therefore, in patients with OSAS, hypoxia results in hearing dysfunctions [29]. Casale et al. compared the auditory data of 18 patients with severe OSAS and 21 patients with a case of simple snoring and showed that the average latencies of I, III and V waves were significantly higher in the OSAS group and declared that the degree and continuance of OSAS alone or together may contribute to decreased neuronal and vascular functions of audio path [19]. In the present study, we found a significantly higher hearing function loss (in 125-4000 Hz and 4000-12000 Hz frequency) in patients with OSAS compared to the control group (Figs. 1 and 2). In the literature, there are studies in which only hearing is handled in patients with OSAS. In Martines et al."s study on patients with mild, moderate and severe OSAS and simple snoring (without OSAS), they evaluated hearing with multi-frequency audiometry and determined that hearing loss in patients with moderate and severe OSAS is higher (41.66%) [30]. Muchnik et al. compared the auditory brainstem responses (ABR) in 79 patients with OSAS and healthy individuals. They found that the transmission duration was expanded in the OSAS group [31]. Kotterba et al. found that OSAS patients had pathologic ABR findings, which show brainstem lesions. They analysed the data of 20 patients with severe OSAS and determined a significant elongation in the first-wave latency and I-V latency in 60% of the total sample [32]. She et al. found the otoacoustic emission (OAE) levels to be significantly lower in patients with OSAS compared to the control group and correlated it to the sensitivity of the cochlea to blood oxygen levels of hair cells [33]. In another study, it was shown that OSAS had various negative effects on hearing and that this hearing impairment might be due to the negative effects of decreased blood oxygen concentrations in both the cochlear sensory epithelium and auditory pathways. [34]. Fu et al. reported that the energy consumption process of the cochlea and neural signal transmission along the auditory path is highly dependent on the blood oxygen source, and therefore, permanent hypoxemia can lead to auditory disorders in OSAS patients. [35]
A limitation of this study is the limited number of patients. A larger group of patients may be necessary in order to have better results.
In conclusion, we suggest that hearing function should be evaluated in OSAS patients and a professional consultation should be sought for OSAS treatment. In addition, serum S100B and NSE measurements may be valued as biochemical indicators in determining hearing loss risk caused by OSAS. We assume that OSAS should be considered in the differential diagnosis of hearing loss and, accordingly, proper treatment planning should be made to prevent further deterioration of hearing function.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
FINANCIAL DISCLOSURE
This study was supported by a research project from the Hatay Mustafa Kemal University, Scientific Research Projects Department (Project Number: 15142)
Reference
1) Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis 2015; 7(5): 920-9.
2) Passali D, Corallo G, Yaremchuk S, Longini M, Proietti F, Passali GC, et al. Oxidative stress in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital 2015; 35(6): 420-5.
3) Hwang JH, Chen JC, Hsu CJ, Liu TC. Association of Obstructive Sleep Apnea and Auditory Dysfunctions in Older Subjects. Otolaryngology-Head and Neck Surgery 2011; 144(1): 114-9.
4) Bradley TD, Floras JS Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009; 373: 82-93.
5) Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: facts and theory. Am J Med 1995; 98: 118-28. [ Özet ]
6) Bloomfield SM, McKinney J, Smith J, Brisman J Reliability of s100B in predicting severity of central nervous system injury. Neurocrit Care 2007; 6: 121-38. [ Özet ]
7) Da Silva LG, Mottin CC, Souza DO, Portela LV, Braga CW, Vargas CB et al. Serum S100B but not NSE levels are increased in morbidly obese individuals affected by obstruc¬tive sleep apnea-hypopnea syndrome. Obes Surg 2008; 18(8): 993-9. [ Özet ]
8) Gonçalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem 2008; 41(10-11): 755-63. [ Özet ]
9) Braga CW, Martinez D, Wofchuk S, Portela LV, Souza DO. S100B and NSE serum levels in obstructive sleep apnea syndrome. Sleep Medicine 2006; 7: 431-5. 10) Spinosi MC, D'Amico F, Passali G, Cingi C, Rodriguez H, Passali D. Hearing loss in mild OSAS and simple snoring patients. Otolaryngol Pol 2017; 30; 71(2): 11-15.
11) Deniz M, Çiftçi Z, Ersözlü T, Gültekin E, Alp R. The evaluation of auditory system in obstructive sleep apnea syndrome (OSAS) patients. Am J Otolaryngol 2016; 37(4): 299-303.
12) American Academy of Sleep Medicine Iber C, Ancoli-Israel S, Chesson A, Quan S For the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 2007 1st ed. Westchester.
13) The Report of an American Academy of Sleep Medicine Task Force Sleep-Related Breathing Disorders in Adults: Recommendations for Syndrome Definition and Measurement Techniques in c Clinical Research. Sleep 1999; 22(5): 667-89.
14) Clark JG. Uses and abuses of hearing loss classification. Asha 1981; 23: 493-500. [ Özet ]
15) Christou K, Kostikas K, Pastaka C, Tanou K, Antoniadou I, Gourgoulianis KI. Nasal continuous positive airway pressure treatment reduces systemic oxidative stress in patients with severe obstructive sleep apnea syndrome. Sleep Medicine 2009; 10: 87-94. [ Özet ]
16) Nazzaro P, Schirosi G, Clemente R, Battista L, Serio G, Boniello E et al. Severe obstructive sleep apnoea exacerbates the microvascular impairment in very mild hypertensives. Eur J Clin Invest 2008; 38: 766-73. [ Özet ]
17) Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 2008; 177:369-75. [ Özet ]
18) Jordan W, Hagedohm J, Wiltfang J, Groeneveld GL, Tumani H, Rodenbeck A. Biochemical markers of cerebrovascular injury in sleep apnoea syndrome. Eur Respir J 2002; 20(1): 158-64. [ Özet ]
19) Casale M, Vesperini E, Potena M, Pappacena M, Bressi F, Baptista PJ. Is obstructive sleep apnea syndrome a risk factor for auditory pathway? Sleep Breath 2012; 16: 413-7
20) Dziewas R, Schilling M, Engel P, Boentert M, Hor H, Okegwo A. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatr 2007; 78: 295-7.
21) Duru S, Fırat IH, Colak N, Ginis Z, Delibası T, Ardıç S. Serum S100B protein: a useful marker in obstructive sleep apnea syndrome. Neurologia i Neurochirurgia Polska 2012; 46, 5: 450-5.
22) Böttiger BW, Möbes S, Glätzer R, Bauer H, Gries A, Bärtsch P et al. Astroglial protein S100 is an early and sensitive marker of hypoxic brain damage and outcome after cardiac arrest in humans. Circulation 2001; 103: 2694-8. [ Özet ]
23) Mussack T, Biberthaler P, Kanz KG, Wiedemann E, Gippner-Steppert C, Jochum M. S100b, sE-selectin and sP-selectin for evaluation of hypoxic brain damage in patients after cardiopulmonary resuscitation pilot study. World J Surg 2001; 25: 539-43.
24) Broderick M, Guilleminault C Neurological aspects of obstructive sleep apnea. Ann NY Acad Sci 2008; 1142: 44-57.
25) Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest 2009; 136: 1668-77. [ Özet ]
26) Colrain IM, Campbell KB. The use of evoked potentials in sleep research. Sleep Med Rev 2007; 11: 277-93.
27) Gupta PP, Sood S, Atreja A, Agarwal D. Evaluation of brain stem auditory evoked potentials in stable patients with chronic obstructive pulmonary disease. Ann Thor Med 2008; 3: 128-34. [ Özet ]
28) Ni D. Auditory brain-stem response in obstructive sleep apnea syndrome. Zhonghua Er Bi Yan Hou Ke Za Zhi 1991; 26: 284-6.
29) Lazarini PR, Camargo AC. Idiopathic sudden sensorineural hearing loss: etiopathogenic aspects. Braz J Otorhinolaryngol 2006; 72: 554-61. [ Özet ]
30) Martines F, Ballacchino A, Sireci F, Mucia M, La Mattina E, Rizzo S et al. Audiologic profile of OSAS and simple snoring patients: the effect of chronic nocturnal intermittent hypoxia on auditory function. Eur Arch Otorhinolaryngol 2016; 273: 1419-24. [ Özet ]
31) Muchnik C, Rubel Y, Zohar Y, Hildesheimer M. Auditory brainstem response in obstructive sleep apnea patients. J Basic Clin Physiol Pharmacol 1995; 6: 139-48. [ Özet ]
32) Kotterba S, Rasche K.. Acoustic evoked potentials (AEP) in obstructive sleep apnea syndrome. Pneumologie 1996; 50: 924-6. [ Özet ]
33) She WD, Zhang Q, Chen F, Jiang P, Wang J. Periuvulopalatopharyngoplasty otoacoustic emissions in patients with obstructive sleep apnea-hypopnea syndrome. Zhonghua Er Bi Yan Hou Ke Za Zhi 2004; 39: 48-51. [ Özet ]




