COMPARISON OF PATIENT POST-OPERATIVE OUTCOMES OBTAINED WITH BIOMATERIALS USED FOR RECONSTRUCTION OF ORBITAL BLOW-OUT FRACTURES
2Liv Hospital Ankara, Plastik, Rekonstrüktif ve Estetik Cerrahi, Ankara, Turkey
3SBÜ Ankara Numune Eğitim ve Araştırma Hastanesi, Plastik, Rekonstrüktif ve Estetik Cerrahi, Ankara, Turkey
4Özel Esentepe Hastanesi, Plastik,Rekonstrüktif ve Estetik Cerrahi, Bursa, Turkey
Summary
Purpose: In the surgical treatment of orbital blow-out fractures, it is essential to restore the orbital volume by proper placement of a biomaterial that can adequately support the orbital content. Many autogenous and synthetic biomaterials have been recommended for this purpose. This study aims to compare postoperative results of 4 different biomaterials.Materials and Method: 64 orbital floor fractures were reconstructed in 62 patients presenting to our clinic with maxillofacial trauma. For reconstruction of orbital floor defects, an iliac bone graft was used in 14 patients, a conchal cartilage graft was used in 19 patients, an ultra thin porous polyethylene implant was used in 15 patients and a titanium mesh was used in 16 patients.
Results: An implant extrusion was observed in 2 of 15 patients in whom a porous polyethylene implant was used. Two patients developed upper gaze limitation and vertical diplopia at this group. Two patients each from the groups with bone and cartilage grafts and one patient from the titanium mesh group had permanent vertical diplopia in extreme gazes. One patient in the titanium mesh group developed an infection that did not require implant excision.
Conclusion: In our study, the complication rate in the conchal cartilage graft and titanium mesh group was significantly lower than other biomaterials. Complications of porous polyethylene implant such as postoperative implant extrusion, enophthalmus persistence, permanent upward gaze restriction suggest that it is not an optimal biomaterial for orbital floor reconstruction. The iliac bone graft often causes donor area complications such as severe pain, hematoma and scar and prolongs the operation. For these reasons, in line with the results of our study; we believe that conchal cartilage graft is the most suitable biomaterial to use for orbital floor defects that are smaller than 4 cm2 and a titanium mesh implant is better for defects that are larger than 4 cm2.
Introduction
Trauma to the face caused by assault or impact from solid objects, often causes internal orbita fractures. In 1957, Smith and Regan [1] described the term "orbital blow-out fracture" from their observation that blunt eyeball trauma due to a tennis ball or a fist increased intraorbital pressure without disruption of soft tissue integrity or causing a fracture line in orbital rims but could cause orbital floor fractures. Such fractures have been categorized as "pure blow-out fractures" in which only the orbita floor is affected, and "impure blow-out fractures" in which fractures of other maxillofacial bones such as zygomatic, maxillary, and nasoethmoid are also affected [2].Physical examination reveals periorbital edema and ecchymosis, subconjunctival hemorrhage, limitation of eye globe movements, diplopia, enophthalmos, dystopia, and infraorbital hypoesthesia (Figure 1) [3]. Enophthalmos is defined as posterior displacement of the eye globe within the orbita where displacement by 2 mm or more is considered to be clinically significant [4].
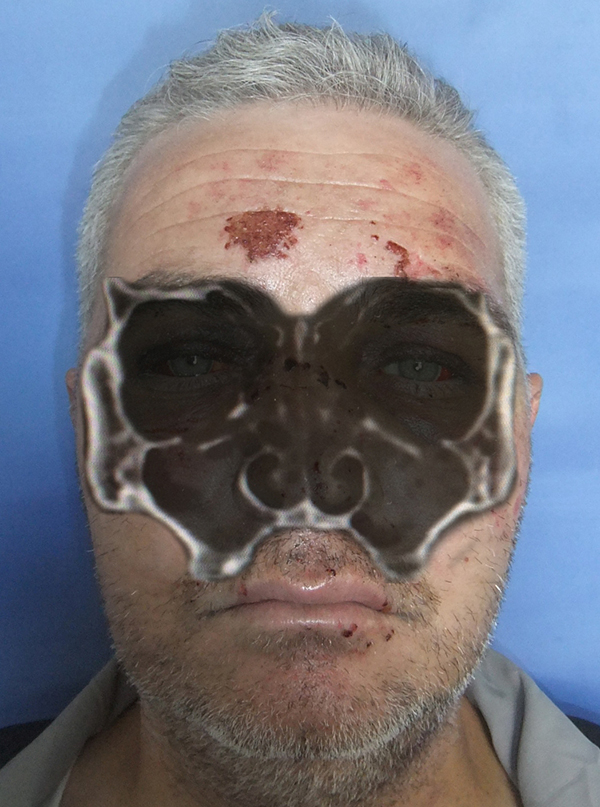 Büyütmek İçin Tıklayın |
Figure 1: Bilayered image of the bilateral blow-out fracture |
The mechanism of enophthalmos and diplopia, especially in vertical gaze, is prolapsus of the orbital contents into the maxillary sinus and their compression at the fracture line. There are many differing views on the indications for surgical repair of orbital floor fractures. Mechanical muscle compression causing diplopia is one of several accepted surgical indications [4]. This situation can be identified using the forced duction test or imaging techniques. Another indication is the persistence of progressive enophthalmos following resolution of trauma-induced edema [4]. Many surgeons believe that defects larger than 1 cm2 require surgery as they cause enophthalmos, but this size limitation is controversial [4].
Reconstruction of the orbital bony structures is of prime importance with respect to preserving normal eye functions and providing an aesthetic view. Although many surgical approaches have been defined in the literature regarding the mode and timing of treatment, no consensus exists [5-7]. Many autogenous and alloplastic biomaterials have been recommended for use to correct orbital bone defects. The autogenous biomaterials used for orbital floor reconstruction include bone, cartilage, and fascia grafts. Resorption of the graft, a long operation time, and donor area morbidity are among factors limiting use of autogenous grafts [8,9]. These drawbacks are especially prominent in bone grafts [9-11]. Cartilage grafts are more easily obtained than bone grafts and are more malleable while exhibiting minimal resorption after implantation [8].
For a partial solution to the inherent disadvantages of autogenous grafts, allogenic materials have also been used to correct orbital floor defects [12-14]. Alloplastic biomaterials used for this indication include implants with a variety of structures, such as silicon, teflon, vitalium, polyethylene, methyl methacrylate, polydioxanone, polyglycolic acid, polylactic acid, polyglactin, bioactive glass, hydroxyapatite and titanium [14,15]. An ideal implant material should be sterilizable, chemically inert, non-allergic, non-carcinogenic, biocompatible, easy-to-remove, malleable, cost-effective, and resistant to deformation and stress. In addition, it should not induce a foreign body reaction or create a medium for growth of microorganisms [4,13]. Problems with the use of any of these materials include infection and extrusion risks.
The aim of the surgical treatment of orbita floor fractures is to free the orbital contents, to cover the defect with an implant material, and to reconstruct the anatomy and orbital volume. The aim of this study is to compare post-operative outcomes of patients presenting with pure and impure blow-out fractures repaired with cartilage, bone grafts, titanium mesh or porous polyethylene implant.
Methods
Sixty-four orbital floor fractures were detected in 62 patients who presented to our clinic with maxillofacial trauma. Forty-seven (76%) of the patients were male and 15 (24%) were female. The age range was 15 to 54 years, with a mean age of 32 years. The cause of the fracture was an assault in 32 patients (52%), an impact by a solid object in 14 patients (22%), a traffic accident in 12 patients (19%), a fall in three patients (5%) and a blast injury in one patient (2%).All patients underwent maxillofacial examination, sinus waters radiography and axial-coronal plane maxillofacial and orbital computerized tomography. Evaluation of the results of the radiographic and physical examinations revealed a unilateral blow-out fracture in 60 of 62 patients and bilateral blow-out fractures in two patients. Of 64 orbita fractures, 26 were pure and 38 were impure blow-out fractures.
A pre-operative ophthalmology consultation was obtained for all patients. Existence of enophtalmos was assessed by an ophthalmologist using a Hertel exophthalmometer. 2 mm or more posterior displacement was stated as enophtalmos. Ocular examination showed diplopia in vertical gaze in 31 patients, diplopia plus enophthalmos in 11 patients, and isolated dystopia in 9 patients. In addition to the results from coronal orbital tomography, the surgical indications were presence of diplopia, dystopia, enophthalmos, and accompanying fracture(s). 11 patients had a normal physical examination but underwent surgery because orbital tomographic images showed a defect larger than 1 cm2 at the orbita floor. The median time from the trauma to the operation was 8 days (range 2-60 days). The clinical approach was to operate on all patients within the first 10 days after the trauma. However, 7 patients were operated more than 15 days after the trauma because of delayed presentation or concurrent medical conditions.
All patients were placed under general anesthesia for surgery. Using suspension sutures, traction was applied on the lower eyelid and using a subciliary incision the muscle-skin flap was elevated and the orbita floor was accessed. The defect was exposed by freeing the orbital contents compressed within the defect as necessary. To reconstruct orbital floor defects, a unicortical bone graft obtained from the iliac wing was used in 14 orbitas, a conchal cartilage graft from the ear was used in 19 orbitas, an ultra thin porous polyethylene sheet (0.85 mm thick) was used in 15 orbitas and a titanium mesh was used in 16 orbitas. The choice of biomaterial to be used was based on the size of the orbital floor defect. Conchal cartilage graft was recommended to patients with defect size less than 4 cm2. If the patient's insurance covers alloplastic material in patients with extensive orbita floor defect porous polyethylene implant or titanium mesh was preferred. The iliac bone graft was preferred in patients with extensive defects who don't have health insurance or whose insurance did not cover the alloplastic material because of the cost of these alloplastic materials.
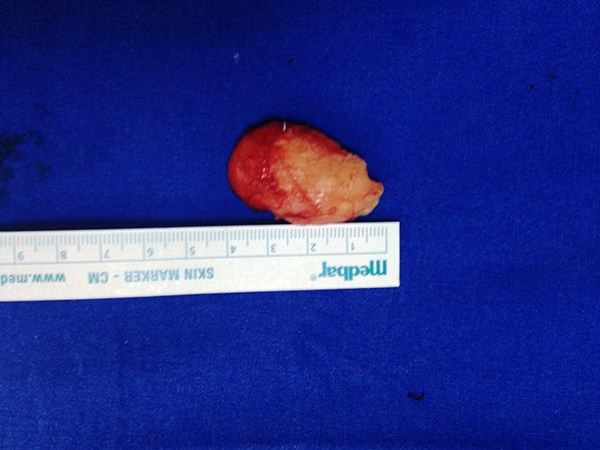
The conchal cartilage graft was harvested from by entering through a posterior incision and adding the posterior perichondrium to the graft (Figure 2). The cartilage graft was placed so the perichondrium faced the maxillary sinus. The titanium mesh implants were fixed to the lower orbital rim using 2 micro screws. Shapes of all autologous and alloplastic implants were modified to fit the defect and orbita floor. After placement of the implant, eye globe movements were tested with the forced duction test. After determining that eye globe movements in all directions were unrestricted, chantopexy was performed to prevent post-operative lower eyelid retraction and then the incision was closed with double layers of 5/0 polyglactin suture. Reduction and plaque-screw fixations of all fractures of other maxillofacial bones accompanying the orbital floor fracture were performed simultaneously with the orbital floor fracture reconstruction.
 Büyütmek İçin Tıklayın |
Figure 2: Harvested cartilage graft |
The suspension sutures in the lower eyelid were removed on the second day after surgery, and the patients were instructed to massage the eye to prevent lower eyelid retraction. The patients were followed post-operatively for 6 to 31 months, with a mean follow-up of 14 months. All patients had ophtalmology consultation at follow-up in order to assessing enophtalmos existence by Hertel exophthalmometer even late postoperative period.
Patients are informed and gave consent to have their picture published in the article.
Results
A permanent, post-operative, vertical diplopia in extreme gazes was detected in 3 of 14 patients in whom the orbita floor was reconstructed with an iliac wing bone graft. However, since eye globe movements were free in all directions in both of these patients, diplopia was considered to be the result of muscle contusion and a second operation was deemed unnecessary. A hematoma requiring drainage developed at the donor area in two patients in this group. All patients in whom a bone graft was taken from the iliac wing complained of severe pain at the donor area. None of the patients developed an infection or experienced implant extrusion.2 of 19 patients who underwent reconstruction using an ear conchal cartilage graft had vertical diplopia in extreme gazes four months after the operation. However, no surgical re-operation was scheduled since eye globe movements were unlimited in all directions. None of the patients in this group developed any donor area complications, infection, or implant extrusion.
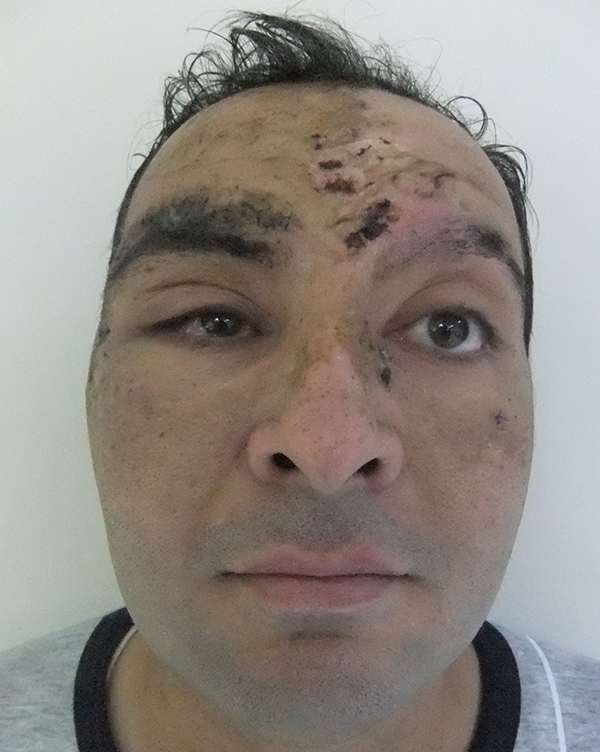
The implant extruded and became palpable in 2 of 15 patients in the porous polyethylene implant group. In one of these patients, the extruded part of the implant was trimmed surgically and the other patient refused reoperation (Figure 3). Two patients suffered from permanent upper gaze limitation and vertical diplopia in functional gazes. One of these patients was reoperated 1 month after the operation because of persistent enophthalmos (Figure 4, 5). During the operation, it was noted that the implant had adhered to the inferior rectus muscle. An iliac bone graft was placed between the inferior rectus and porous polyethylene implant to reduce the volume of the orbita after freeing the implant from the muscle. Post-operatively, the upper gaze limitation was improved and enophthalmos was reduced. However, diplopia in straight gaze became permanent, perhaps because of incomplete improvement of enophthalmos. The other patient with persistent upper gaze limitation underwent another operation two months after the first operation. During the second operation the adhesions between the porous polyethylene implant and the inferior rectus muscle were removed and, to prevent future adhesions, a tensor fascia lata graft was placed between the inferior rectus muscle and the implant. After the operation, the upper gaze limitation improved but vertical diplopia persisted in functional gazes, perhaps because of inferior rectus injury. None of the patients in this group developed infection.
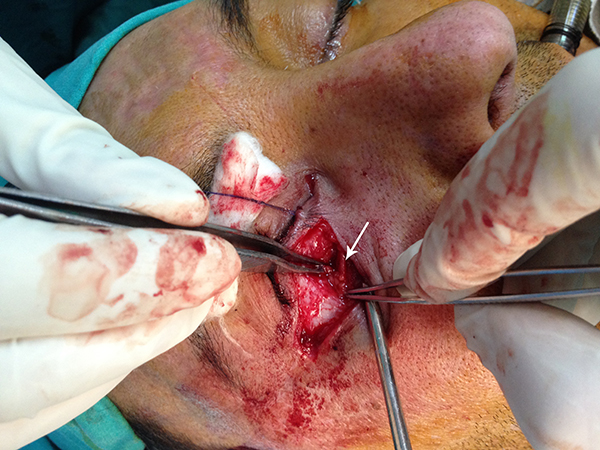 Büyütmek İçin Tıklayın |
Figure 3: Extrusion of the porous polyethylene implant |
 Büyütmek İçin Tıklayın |
Figure 4: Preoperative image of the patient with severe right enophtalmus who was operated 1 months after the trauma because of the concurrent medical conditions. |
 Büyütmek İçin Tıklayın |
Figure 5: Persistent right enophtalmus 1 months after the operation |
In the titanium mesh group, 1 of 11 patients had permanent, post-operative vertical diplopia in extreme gazes. A repeat operation was not considered to be necessary in that patient. A post-operative infection developed in one diabetic patient in whom the orbita floor was completely defective and was reconstructed with a titanium mesh. That patient underwent reduction and fixation with a plaque-screw from the oral cavity for a fragmented fracture in the zygomaticomaxillary region at the same time. A purulent discharge was drained from the maxillary sinus. The site of infection was irrigated with antibiotics regularly and was treated with intravenous antibiotic therapy. The infection regressed and it was not necessary to remove the titanium mesh. No implant extrusion was observed in any of the patients in this group. (Figure 6, 7)
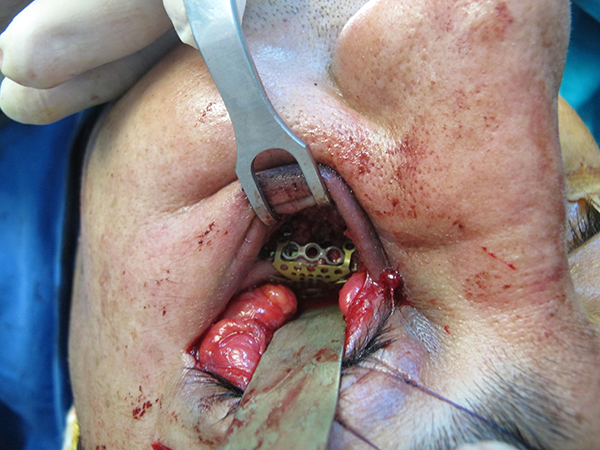 Büyütmek İçin Tıklayın |
Figure 6: The titanium mesh placed to the orbital floor |
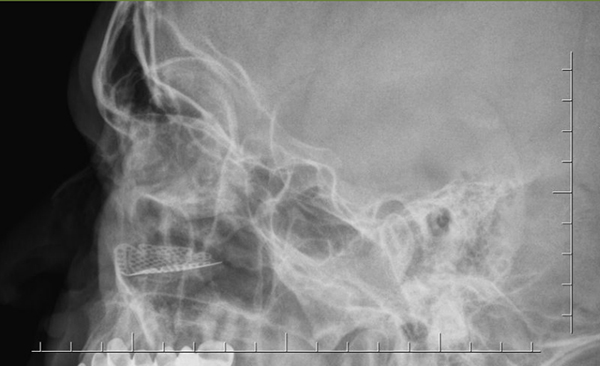 Büyütmek İçin Tıklayın |
Figure 7: Radiographic image of the implanted titanium mesh |
Enophtalmos was persisted only 1 patient postoperatively among 11 patients who had preoperative enophtalmos. This patient was operated 1 months after the trauma because of the concurrent medical conditions. Postoperative period of this patient whose orbital floor was reconstructed with porous polyethylene is mentioned before in the text. None of the patients in the iliac bone and conchal cartilage autograft groups was presented late postoperative enopthtalmos according to the graft resorption (Figure 8) (Table 1).
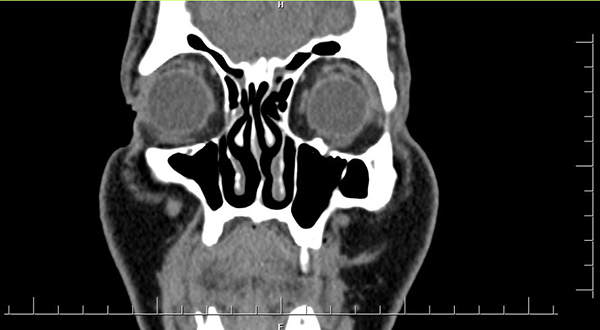 Büyütmek İçin Tıklayın |
Figure 8: Maxillofacial CT image of the left orbital bone graft postop. 6. Months |
Table 1: Postoperative results of the four biomaterial groups
Discussion
There is no consensus in literature as to the best mode and timing of reconstruction of internal orbital fractures [16-18]. Many authors recommend use of different synthetic and autogenous biomaterials, each with its own advantages and disadvantages, for reconstruction of orbital floor defects [15-18]. In the past, there was a common belief that bone grafts were the most appropriate implant materials for orbital floor reconstruction [4,18]. Autogenous bone grafts have been recommended by virtue of their ability to provide a more sustained and stable reconstruction. Bone graft donor areas include iliac crest, ribs, calvarium, anterior wall of maxilla, and mandible [14].The iliac bone grafts used in our study are applicable to orbital floor reconstructions; however, they have some disadvantages, including the long time required for dissection, the risk of hematoma and pain at the donor area, visible donor area scar and a more prominent resorption in the graft due to its enchondral origin [4,14]. In addition, it is not possible to shape the bone tissue to fit to the contour of the orbital floor because of its rigid structure.
The advantages of ear conchal cartilage are evident because the donor area is next to the operative field and harvesting the graft is technically easier and, since the scar is behind the ear, donor area morbidity is negligible. The concave shape of the concha is appropriate for the anatomy of the orbita floor, and its elastic and malleable structure facilitates adaptation of the cartilage graft to the defect [10,19-21]. Furthermore, resorption of cartilage grafts occurs less frequently than resorption of bone grafts [14]. In addition, the cartilage grafts increase mucosal regeneration when the perichondrium faces the maxillary defect [8].
With all their advantages, and their only disadvantage being a size limitation, conchal cartilage grafts are underutilized in orbital floor reconstructions. A meta-analysis published in 2013 examined 4 studies of conchal cartilage grafts in orbita floor reconstruction among twenty-three patients. Six patients had persistent diplopia and 2 had persistent post-operative enophthalmos [13,19,20,22-26]. The same meta-analysis examined twenty-one studies in which autogenous grafts were used to repair orbital floor fractures and found no patients developed graft extrusion, displacement, or infection [13]. Those who received a cartilage graft had the lowest complication rate in our study. The complications in the bone graft group occurred primarily in the donor area. The results allow us to conclude that a cartilage graft is a more suitable autogenous material than a bone graft for orbital floor reconstruction in moderately large defects.
Alloplastic materials are easy to obtain and are not subject to resorption. Synthetic materials are easy to use but their costs and risks are debatable. Infections, extrusion of the material from the skin, or displacement of the material are the most common complications when synthetic materials are used to repair orbital blow-out fractures [27-29]. Among the alloplastic materials we used in our study, the highest complication rate was observed with the porous polyethylene implant because it allows ingrowth of adjacent tissues into the graft material. Although this is important for stabilization, it can lead to adhesion of the implant to the rectus muscle if the inferior rectus sheath is damaged. Consequently, upper gaze limitation and diplopia can develop as found in 2 patients in this group. This risk of complication means that a porous polyethylene implant is not suitable for orbital floor reconstruction in patients with a damaged inferior rectus sheath. In addition, properties of the porous polyethylene implants do not prevent implant extrusion since the implants were extruded in 2 of these patients. To avoid this particular complication, a porous polyethylene implants works better if it is fixed to the orbital floor with a screw. Of the 15 patients with porous polyethylene implants, reoperation was indicated in 4 patients (26,6%). 2 of them due to upper gaze limitation, 2 of them due to implant extrusion. Among these 4 patients, 3 patients who accept surgery were re-operated. 26.6% reoperation indication rate in porous polyethylene implant group is a quite high rate. No other patient required reoperation in the other 3 groups. This suggests that the porous polyethylene implant is not an appropriate choice of alloplastic material in orbital floor reconstruction.
Among alloplastic materials, the best results were obtained when titanium mesh was used. The most commonly used material, titanium has the greatest tensile strength despite being easily bent and it is the least corrosive of the metals. Often used in facial bones, titanium has high biocompatibility with a low risk of infection, and further, it is compatible with radiographic imaging [30-32]. Titanium mesh is advantageous because of its high level of backup and malleability, which allows it to fit large defects or defects involving the medial side of the orbital floor [10,30,33-35]. Gear et al. reported good functional outcomes and minimal risk of infection in a 44-month follow-up study where orbital floor defects larger than 2 cm2 were reconstructed with titanium mesh in fifty-five patients [30]. Other studies where titanium mesh was used have similarly reported no or minimal post-operative infection [30,36,37]. We encountered no complications with the use of titanium mesh with the exception of 1 patient who suffered from an infection. Synthetic titanium mesh was preferred especially in patients with a large orbital floor defect in whom a cartilage graft could not be used (Table 2).
Table 2: Biomaterials and their properties
The most common complications after surgical repair of orbital fractures include lower eyelid retraction and enophthalmos, which is usually related to an increase in orbital volume resulting from improper placement of the implant material on the orbital floor [4,38,39]. Since the orbital floor has a posterior cephalic slope, the implant must be placed in an appropriate anatomical position to fit precisely [4]. If not, the implant can displace into the maxillary sinus, resulting in an increase in orbital volume and enophthalmos, which is often resistant to corrective surgical interventions [4]. For these reasons, how the implant is placed is more important than the type of implant material that is placed. Effort should be made to restore the orbita volume and contour. It is mandatory to test eye globe movements with the forced duction test after placement of the implant.
Results show that the ear conchal cartilage graft was the best biomaterial used to repair defects smaller than 4 cm2. It is an easy-to-use biomaterial that fits the anatomical shape of the orbital floor, resulting in minimal donor area morbidity, lower treatment costs, and satisfactory post-operative patient outcomes. Among the synthetic materials tested, titanium mesh was a good option to repair defects larger than 4 cm2. It provides sufficient strength, is easy to use, and is associated with a low rate of post-operative complications and favorable patient outcomes. However, selection of the optimal biomaterial to be used to repair orbital blow-out fractures should be made according to patient characteristics and preoperative findings, the severity of the injury, the cost of the biomaterial to be used, and surgeon's expertise.
Conflict of interest:
The authors declare that there is no conflict of interest regarding the publication of this article.
Reference
1) Smith B, Regan WF Jr. Blow-out fracture of the orbit: Mechanism and correction of internal orbital fracture. Am J Ophthalmol 1957, 44:733. [ Özet ]
2) Smith B, Converse JM. Early treatment of orbital floor fractures. Trans Am Acad Ophthalmol Otolaryngol 1957, 61:602, 1957 [ Özet ]
3) Scherer M, Sullivan WG, Smith DJ Jr, et al. An analysis of 1423 facial fractures in 788 patients at an urban trauma center. J Trauma 1989, 29:388. [ Özet ]
4) Hollier LH Jr, Kelley P. Soft tissue and skeletal injuries of the face. In: Thorne CH, Beasley RW, eds. Grabb and Smith's Plastic Surgery. 6th ed. Philadelphia: Lippincott Williams and Wilkins. 2006: 315-331
5) Wilkins RB, Havins WE. Current treatment of blow-out fractures. Ophthlamology 1982;89:464-6. [ Özet ]
6) Patel BC, Hoffman J. Management of complex orbital fractures. Facial Plast Surg 1998;14:83-104. [ Özet ]
7) Burnstime MA. Clinical recommendations for repair of isolated orbital floor fractures: an evidence-based analysis. Ophthalmology 2002;109:1207-10. [ Özet ]
8) Constanitian MB. Use of auricular cartilage in orbital floor reconstruction. Plast Recons Surg 1982; 69:951. [ Özet ]
9) Stevenson S. Biology of bone grafts. Orthop Clin North Am 1999, 30:543. [ Özet ]
10) Glassman RD, Manson PN, Vanderkolk CA, Iliff NT, Yaremchuk MJ, Petty P, et al. Rigid fixation of internal orbital fractures. Plast Reconstr Surg 1990;86:1103-11. [ Özet ]
11) Philips JH, Rahn BA. Fixation effect on membranous and endochondral onlay bone graft revascularization. Plast Reconst Surg 1990 Jun;85(6):891-7. [ Özet ]
12) Lipschitz AH, Kenkel JM. Implantation: Bone, cartilage and alloplasts.Selected Readings in Plastic Surgery 2004. Vol. 10. 1-25
13) Gunarajah DR, Samman D. J. Biomaterials for repair of orbital floor blowout fractures: a systematic review. Oral Maxillofac Surg 2013 Mar;71(3):550-70. [ Özet ]
14) Baino F. Biomaterials and implants for orbital floor repair. Acta Biomater 2011 Sep;7(9):3248-66. [ Özet ]
15) Burres SA, Cohn AM, Mathog RH. Repair of orbital blow-out fractures with Marlex mesh and Gelfilm. Laryngoscope 1981; 91: 1881-6. [ Özet ]
16) Potter JK, Ellis E. Biomaterials for the reconstruction of the internal orbit. J Oral Maxillofac Surg 62:1280, 2004. [ Özet ]
17) Koornef L. Current concepts on the management of blowout fractures. Ann Plast Surg 1982; 9:185-200.
18) Chowdhury K, Krause GE. Selection of materials for orbital floor reconstruction. Arch Otolaryngol Head Neck Surg 1998;124:1398-401. [ Özet ]
19) Talesh KT, Babaee S, Vahdati SA, Tabeshfar S: Effectiveness of a nasoseptal cartilaginous graft for repairing traumatic fractures of the inferior orbital wall. Br J Oral Maxillofac Surg 2009, 47:10. [ Özet ]
20) Ozyazgan I, Eskitascioglu T, Baykan H, Coruh A: Repair of traumatic orbital wall defects using conchal cartilage. Plast Reconstr Surg 2006, 117:1269. [ Özet ]
21) Hendler BH, Smith BM. Use of auricular cartilage in the repair of orbital floor defects. Oral Surg Oral Med Oral Pathol 1992, 74:719. [ Özet ]
22) Kinnunen I, Aitasalo K, Pollonen M, Varpula M: Reconstruction of orbital floor fractures using bioactive glass. J Craniomaxillofac Surg 2000, 28:229-34. [ Özet ]
23) Kraus M, Gatot A, Fliss DM. Repair of traumatic inferior orbital wall defects with nasoseptal cartilage. J Oral Maxillofac Surg 2001, 59:1397. [ Özet ]
24) Lai A, Gliklich RE, Rubin PAD. Repair of orbital blow-out fractures with nasoseptal cartilage. Laryngoscope 1998, 108:645. [ Özet ]
25) Castellani A, Negrini S, Zanetti U. Treatment of orbital floor blowout fractures with conchal auricular cartilage graft: A report on 14 cases. J Oral Maxillofac Surg 2002, 60:1413. [ Özet ]
26) Bayat M, Momen-Heravi F, Khalilzadeh O, et al. Comparison of conchal cartilage graft with nasal septal cartilage graft for reconstruction of orbital floor blowout fractures. Br J Oral Maxillofac Surg 2010, 48:617. [ Özet ]
27) Wolfe SA. Correction of a lower eyelid deformity caused by multiple extrusion of alloplastic orbital floor implants. Plast Reconst Surg 1969; 43:591.
28) Kohn R, Romano PE, Puklin JE. Lacrimal obstruction after migration of orbital floor implant. Am J Opthalmol 1976; 82:934-6. [ Özet ]
29) Alpar JJ. Unusual complication of orbital blow-out fracture repair. Ann Opthalmol 1977; 9:1173-4. [ Özet ]
30) Gear AJ, Lokeh A, Aldrifge JH. Safety of titanium mesh for orbital reconstruction. Ann Plast Surg 2002, 48:1. [ Özet ]
31) Albrektsson T, Branemark PI, Hansson HA, Lindstrom J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 1981, 52:155. [ Özet ]
32) Schubert W, Gear AJ, Lee C, Hilger PA, Haus E, Migliori MR, et al. Incorporation of titanium mesh in orbital and midface reconstruction. Plast Reconstr Surg 2002;110:1022-32. [ Özet ]
33) Metzger MC, Schon R, Schulze D, et al. Individual preformed titanium meshes for orbital fractures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006, 102:442. [ Özet ]
34) Shetty P, Kumar SG, Baliga M, Uppal N. Options in orbital floor reconstruction in blowout fractures: a review of ten cases. J Maxillofac Oral Surg 2009, 8:137-40. [ Özet ]
35) Wang S, Xiao J, Liu L, Lin Y, Li X, Tang W, et al. Orbital floor reconstruction: a retrospective study of 21 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:324-30. [ Özet ]
36) Ellis E, Tan Y. Assessment of internal orbital reconstruction for pure blowout fractures: cranial bone versus titanium mesh. J Oral Maxillofac Surg 2003;61:442-53. [ Özet ]
37) Mackenzie DJ, Arora B, Hansen J. Orbital floor repair with titanium mesh screen. J Craniomaxillofac Trauma 1999;5:9-18. [ Özet ]
38) Broyles JM, Jones D, Bellamy J, Elgendy T, Sebai M, Susarla SM et al. Pediatric Orbital floor fractures: Outcome Analysis of 72 Children with Orbital Floor Fractures. Plast Reconstr Surg 2015; 136(4):822-8. [ Özet ]
39) Peacock ZS, Boulos T, Miller JB, Gardiner MF, Chang SK,Troulis MJ. Orbital fractures and ocular injury: Is a postoperative ophthalmology examination necessary? J Oral Maxillofac Surg. 2014;78:1533-1540. [ Özet ]




