TENASCIN EXPRESSION PATTERNS OF SALIVARY GLAND TUMORS: AN IMMUNHISTOCHEMICAL AND COMPARATIVE STUDY
2Kocaeli University Faculty of Medicine, Department of Otorhinolaryngology, Kocaeli, Turkey
Summary
Purpose: Tenascin is a multifunctional large glycoprotein component of extracellular matrix. Salivary gland neoplasms present a most diverse group of tumors some of which are rich in extracellular matrix. The study was set out to evaluate the expression of tenascin in a series of salivary gland neoplasms.Materlals and Methods: 32 salivary gland tumors including; 18 pleomorphic adenomas, 8 Warthin’s tumors, 2 basal cell adenoma, 1 acinic cell carcinoma, 2 adenoid cystic carcinoma, 1 malignant pleomorphic adenoma were included in this study. All of the cases were stained with tenascin with immunohistochemical method. The staining intensities and localizations were evaluated.
Results: In pleomorphic adenomas tenascin expression was intensive in tumoral stromal component especially around chondroid islands. In Warthin’s tumor staining was usually observed beneath epithelial regions and in the epithelial component.
Conclusions: The main source of tenascin in salivary gland tumors is tumor’s stromal component or stroma. The major role of tenascin in pleomorphic adenoma may be proliferation and chondroid differentiation of stromal component. In Warthin’s tumor, tenascin observed under the epithelial component may be responsible of epithelial and mesenchymal interaction and high ratio of intracytoplasmic epithelial tenascin expression. This could be related with the cytokine secretion from the lymphoid stroma
Introduction
Tenascin is a multifunctional large glycoprotein of the extracellular matrix, which is found in embryonic tissues, especially in areas of epithelial mesenchymal junctions. It probably has a role in epithelial-mesenchymal induction and cell migration [1-7]. Tenascin expression is observed in healing wounds, in dense connective, cartilaginous, smooth muscle tissue and in basement membranes [4,5,6,8,9]. Its immunoreactivity has been reported in a wide variety of benign and malignant neoplasms including salivary gland tumors [10-20].Salivary gland neoplasms present a most diverse group of tumors. They originate from the cells of neuroectodermal or neural crest which have the potential of differentiation to a wide variety of cell types [16,21]. Immunoreactivity of tenascin has been reported in these tumors, suggesting having a role in stromal remodeling [22].
We carried out the present immunohistochemical study to elucidate the presence of tenascin in salivary gland tumors including rare variants in order to observe its possible role in tumor progression.
Methods
The tumor series were retrieved from the surgical pathology files of our Pathology Department. Clinical information was obtained from the patients records. The review spanned the period from 1996 to 2002. 32 cases of salivary gland tumors were examined including 18 cases of pleomorphic adenoma, 8 cases of Warthin’s tumor, 2 cases of basal cell adenoma, 1 case of acinic cell carcinoma, 2 cases of adenoid cystic carcinoma, 1 cases of malignant pleomorphic adenoma.One representative tissue block was selected from each tumor for immunohistochemical evaluation. 4ìm sections were cut from each paraffin-embedded tissue block. The sections were deparaffinized washed with tap water and boiled in citrate buffer saline for 20 min. for antigen retrieval. This procedure was followed by application of the primary antibody to tenascin (TN2 DAKO Carpenteria CA) in 1/50 dilution. Antibody detection was performed by adding biotin layered secondary antibodies, avidin-biotin complex and 3, 3’-diaminobenzidine. Negative controls were run in non immune serum and positive controls with appropriate tissue.
Immunoreactivity was evaluated in reference to the expression site, and intensity of tenascin. The evaluation according to the immunoreactivity localization showed some differences in different types of tumors. Epithelial, myoepithelial, stromal and intraluminal immunoreactivity were evaluated in pleomorphic adenomas Warthin’s tumors, adenoid cystic carcinomas, and malignant pleomorphic adenomas. The outer layer of the tumoral islands was accepted as myoepithelial, gland forming inner component accepted as epithelial. In basal cell adenomas epithelial and myoepithelial component identification was not possible. Only epithelial component was observed and evaluated in acinic cell carcinomas. The staining intensity was graded as; (+): focal and faint staining, (+++): immunoreactivity present in more than 75% of the slide, and (++) staining between these two intensity.
Results
Localizations and mean diameters of different salivary gland tumor are summarized in Table 1. Percentages associated with tenascin expression intensity and localization was summarized in Table 2.Pleomorphic adenomas were from 8 females and 10 males, the age range was 14 to 68 years. Tumor diameters were ranged between 1.8 and 5.0 cm. All tumors except the one from submandular gland were originated from the parotid gland. All Warthin’s tumors were in males with an age range between 38 and 72. In one case there were two tumors in the same gland, and in another there were separate tumors in each parotid gland. Adenoid cystic carcinomas originated from hard palate and from the base of tongue. Acinic cell carcinoma originated from the submandibular gland of a 21 year old woman. The malignant pleomorphic adenoma tumor was of a 62 year old male, who had tumor recurrence.
Tenascin expression was observed in all tumors and in nontumoral salivary gland. Tenascin expression was observed in epithelial and stromal interactions of acinar and ductal structures in normal salivary ducts.
Intensity of tenascin expression showed differences in tumor groups. The most intensive expression was observed in pleomorphic adenomas. Vascular structures and tumor capsules were also immunoreactive to tenascin in most of the cases.
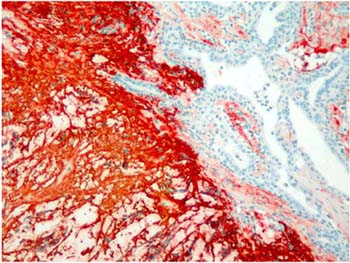
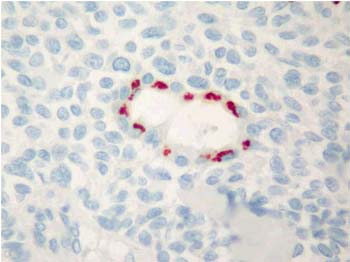
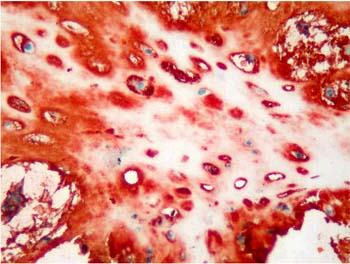
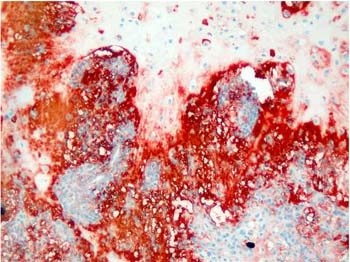
Majority of the pleomorphic adenomas had strong tenascin immunostaining. Tumors rich in stromal component had more intensive and diffuse staining and immunoreactivity was more prominent in stroma than the other components (Fig. 1). Intracytoplasmic clustures were often observed in the epithelial component (Fig. 2). Stromal immunoreactivity was stronger in myxoid areas and weaker in hyalinised areas and chondroid matrix was always immunoreactive. Chondrocytes stained strongly and an intensive immunoreactive halo was observed around some of them. A strongly staining region in the periphery of chondroid islands was observed in some of cases (Fig. 3, 4). Tenascin immunoreactivity made a chicken wire like pattern around the myoepithelial cell clustures (Fig. 5).
 Büyütmek İçin Tıklayın |
Figure 1: Strong and diffuse stromal immunoreactivity in pleomorphic adenoma. (immunohistochemistry tenascin X40) |
 Büyütmek İçin Tıklayın |
Figure 2: Glandular intracytoplasmic tenascin immunoreactivity in the form of clusters in pleomorphic adenoma. (immunohistochemistry tenascin X400) |
 Büyütmek İçin Tıklayın |
Figure 3: Diffuse immunoreactivity in condroid matrix and intensive immunoreactivity in condrocyte cytoplasms in pleomorphic adenoma. (immunohistochemistry tenascin X200) |
 Büyütmek İçin Tıklayın |
Figure 4: Intensive tenascin immunoreactivity around condroid islands in plemorphic adenoma. . (immunohistochemistry tenascin X40) |
 Büyütmek İçin Tıklayın |
Figure 5: Tenascin immunoreactivity around myoepithelial cells made a pattern like chicken wire in pleomorphic adenoma. (immunohistochemistry tenascin X400) |
In Warthin’s tumor tenascin expression was focal and faint. Staining was observed usually in basal membrane. Intracytoplasmic epithelial globules were observed in oxyphilic cells (Fig. 6). Immunoreactivity of myoepithelial layer was also observed in only one case.
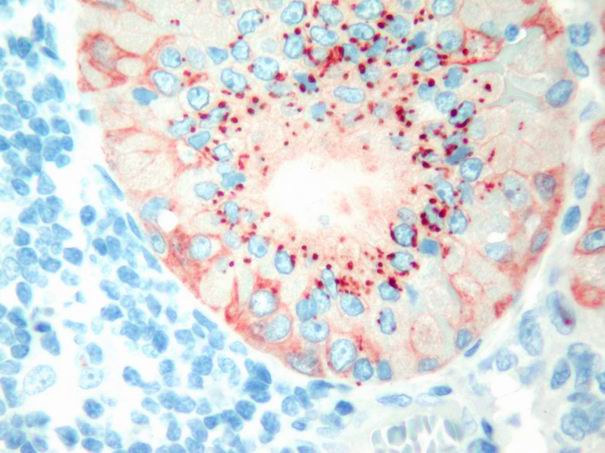 Büyütmek İçin Tıklayın |
Figure 6: Tenascin immunoreactivit in the forms of Intraepithelial globules, and diffuse myoepithelial staining in Warthin’s tumour. (immunohistochemistry tenascin X200) |
In the basal cell adenomas, immunoreactivity was weak and detected only in tumor stroma, but not in tumor cells (Fig. 7). In acinic cell carcinoma, immunoreactivity was weak and limited to the stroma. In adenoid cystic carcinomas, focal and faint epithelial and stromal immunoreactivity and prominent intraluminal expression was observed (Fig. 8). In malignant mixed tumor, focal stromal and chondroid immunoreactivity was observed.
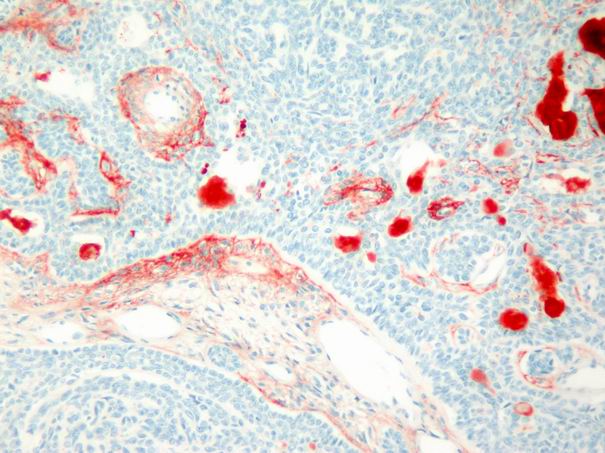 Büyütmek İçin Tıklayın |
Figure 7: Weak stromal and intraluminal tenascin immunoreactivity in basal cell adenoma. (immunohistochemistry tenascin X100) |
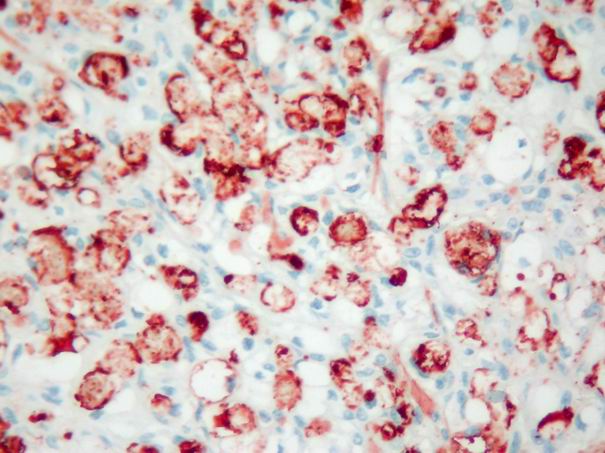 Büyütmek İçin Tıklayın |
Figure 8: Intensive and diffuse intraluminal tenascin expression in adenoid cystic carcinoma. (immunohistochemistry tenascin X400) |
Discussion
We analyzed the distribution of tenascin in normal salivary gland, different kinds of benign and malignant salivary gland tumors and found differences in staining intensity and localisation. In normal salivary gland tenascin was expressed in the same intensity and localization of epithelial mesengial interactions of ductal and aciner system. Tenascin expression in pleomorphic adenoma is significantly higher than that of other salivary gland tumors. Intraepithelial tenascin expression observed in pleomorphic adenoma, Warthin’s tumor, and adenoid cystic carcinoma but it was not present in basal cell adenomas and acinic cell carcinomas. We think that, tenascin may play different roles in tumoral growth in different salivary gland tumors.The effect of tenascin in tumor growth is a well known phenomenon. In tumoral tissues neoplastic cells secret cytokines such as transforming growth factor-beta which stimulates stromal fibroblasts to produce tenascin [7,10,16,23-26]. Tenascin contains epidermal growth factor like repeats and might induce tumor growth by an autocrine mechanism [27]. It seems likely that there is an interaction between stroma and tumoral cells in tenascin expression. This interaction may have specialities in tumors with stromal or lymphoid component.
Pleomorphic adenomas has a complex histopathological morphology with epithelial component containing luminal tumor cells and outer tumor cells termed as modified myoepithelial cells. Myoepithelial cells are responsible for the production of excessive amounts of basal lamina-associated proteins and glycosaminoglycans that can form a variety of stromal components ranging from fibrous, hyaline, myxoid and chondroid [28,29]. Myoepithelial cells that have capacity to differentiate in various stromal components are the cause of the pleomorphic appearance of this neoplasia.
Strong immunoreactivity of tenascin in stromal component suggests a major role of this protein in the progress of in pleomorphic adenoma. The tenascin secretion from the stromal component modified from myoepithelial cells may cause proliferation of myoepithelial cells and myoepithelial cells produce more stroma. This positive feed-back mechanism may cause excessive tenascin accumulation of pleomorphic adenoma. Intensive immunoreactivity of tenascin in malignant and benign pleomorphic adenomas was also reported previously [4,5,11,16,20,30,31].
The condroid changes in pleomorphic adenoma similar to normal hyaline cartilage at the immunohistochemical and ultrastructural level [16,30]. Tenascin has a major role in chondrogenesis and osteogenesis during embriyogenesis and may have a similar function in the stromal component of pleomorphic adenoma [11,16]. It is present diffusely in embyologic chondroid tissue and observed mainly in the adult pericondrial tissue, which seems to be associated with chondroid change [4,32]. We observed more intense immunoreactivity in myxoid rims surrounding the chondroid islets and strongly immunoreactive hallos around the chondrocytes. This also confirmes that tenascin is more intensive during the process of chondroid differentiation and seems to decrease progressively during the maturation process.
We observed a clear difference between the intensity of tenascin immunoreactivity of pleomorphic adenomas and other salivary gland tumors. Except asinus cell carcinoma all the other salivary gland tumors may show S-100 and smooth muscle actin immunoreactivity and known to have myoepithelial differentiation [33]. The stromal component originating from myoepithelial cells is the main difference of pleomorphic adenoma. We also observed less tenascin immunoreactivity in myoepithelial component compared with the stromal component. As a result we strongly suggest that the major source of tenascin in salivary gland tumors were not myoepithelial cells but stromal cells.
On the other hand epithelial tenascin immunoreactivity was observed in Wartin’s tumor, pleomorphic adenoma and faintly in adenoid cystic carcinoma. As it is reported before the tenascin immunoreactivity observed in epithelial and stromal interaction in Warthin’s tumor but intraepithelial immunoreactivity is also reported before [4,13,16,34]. The tumors rich in epithelial component with scanty stroma such as acinic cell carcinoma and basal cell adenoma do not have epithelial tenascin expression. In Warthin’s tumor, tenascin expression beneath the epithelial and stromal component may be responsible for epithelial and mesengial remodeling. The epithelial tenascin secretion may be related with the excessive expression of cytokins in the lymphoid stroma of Warthin’s tumor.
The tenascin expression of salivary gland tumors is dominantly located in the stromal component but may be observed in the intraepithelial region of stromal rich tumors. The amount of the tenascin expression is directly related with the amount of the stromal component and most intensive in the pleomorphic adenoma that has a tumoral stromal component. The major role of the tenascin expression in the pleomorphic adenoma may be the organization of the chondroid matrix. In Warthin’s tumor, the tenascin expression observed beneath epithelial stromal component may be responsible for the epithelial-mesengial organization. Lastly, intraepithelial tenascin expression can be observed in salivary gland tumors with prominent stroma and lymphoid stroma. This may be related with the cytokins secreted from stromal and lymphoid component.
Reference
1) Chiquet M, Fambrough DM. Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J Cell Biol 1984;98:1926-36. [ Özet ]
2) Crossin KL, Hoffman S, Grumet M, Thiery JP, Edelman GM. Site-restricted expression of cytotactin during development of the chicken embryo. J Cell Biol 1986;102:1917-30. [ Özet ]
3) Chuong CM, Crossin KL, Edelman GM . Sequential expression and differential function of multiple adhesion molecules during the formation of cerebellar cortical layers. J Cell Biol 1987;104:331-42. [ Özet ]
4) Soini Y, Paakko P, Virtanen I, Lehto VP. Tenascin in salivary gland tumours.Virchows Arch A Pathol Anat Histopathol 1992;421:217-22. [ Özet ]
5) Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 1986;47:131-9. [ Özet ]
6) Erickson HP, Bourdon MA. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol 1989;5:71-92. [ Özet ]
7) Chiquet-Ehrismann R. What distinguishes tenascin from fibronectin? FASEB 1990;J4:2598-604. [ Özet ]
8) Chuong CM, Chen HM. Enhanced expression of neural cell adhesion molecules and tenascin (cytotactin) during wound healing.Am J Pathol 1991;138:427-40. [ Özet ]
9) Natali PG, Nicotra MR, Bigotti A, Botti C, Castellani P, Risso AM, et al. Comparative analysis of the expression of the extracellular matrix protein tenascin in normal human fetal, adult and tumor tissues. Int J Cancer 1991;47:811-6. [ Özet ]
10) Howeedy AA, Virtanen I, Laitinen L, Gould NS, Koukoulis GK, Gould VE. Differential distribution of tenascin in the normal, hyperplastic, and neoplastic breast. Lab Invest 1990;63:798-806. [ Özet ]
11) Mackie EJ, Thesleff I, Chiquet-Ehrismann R. Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol 1987;105:2569-79. [ Özet ]
12) Tiitta O, Wahlstrom T, Paavonen J, Linnala A, Sharma S, Gould VE, et al. Enhanced tenascin expression in cervical and vulvar koilocytotic lesions. Am J Pathol 1992
;141:907-13. [ Özet ]
13) Natali PG, Zardi L. Tenascin: a hexameric adhesive glycoprotein.Int J Cancer 1989 ;Suppl 4:66-8. [ Özet ]
14) Lightner VA, Gumkowski F, Bigner DD, Erickson HP. Tenascin/hexabrachion in human skin: biochemical identification and localization by light and electron microscopy. J Cell Biol 1989;108:2483-93. [ Özet ]
15) Sakai T, Kawakatsu H, Hirota N, Yokoyama T, Sakakura T, Saito M. Specific expression of tenascin in human colonic neoplasms. Br J Cancer 1993;67:1058-64. [ Özet ]
16) Shrestha P, Sakamoto F, Takagi H, Yamada T, Mori M. Enhanced tenascin immunoreactivity in leukoplakia and squamous cell carcinoma of the oral cavity: an immunohistochemical study.Eur J Cancer B Oral Oncol 1994;30B:132-7. [ Özet ]
17) Bourdon MA, Wikstrand CJ, Furthmayr H, Matthews TJ, Bigner DD. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res 1983;43:2796-805. [ Özet ]
18) Herlyn M, Graeven U, Speicher D, Sela BA, Bennicelli JL, Kath R, Guerry D 4th. Characterization of tenascin secreted by human melanoma cells. Cancer Res 1991;51:4853-8. [ Özet ]
19) Natali PG, Nicotra MR, Bartolazzi A, Mottolese M, Coscia N, Bigotti A, Zardi L. Expression and production of tenascin in benign and malignant lesions of melanocyte lineage. Int J Cancer 1990;46:586-90. [ Özet ]
20) Sunardhi-Widyaputra S, Van Damme B. Immunohistochemical staining of tenascin in Warthin's tumor and in oncocytoma. Oral Surg Oral Med Oral Pathol 1993;76:325-9. [ Özet ]
21) Mackie EJ, Tucker RP, Halfter W, Chiquet-Ehrismann R, Epperlein HH. The distribution of tenascin coincides with pathways of neural crest cell migration. Development 1988;102:237-50. [ Özet ]
22) Sato M, Yoshida H, Azuma M. 5-Azacytidine induction of neuron-like cells from a neoplastic human salivary intercalated duct line and effect of dibuthyryl cyclic adenosine 3’-5’ monophosphate on proliferation and phenotype of induced cell. Cancer J 1993;6:26-36. [ Özet ]
23) Pearson CA, Pearson D, Shibahara S, Hofsteenge J, Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-beta.EMBO J 1988; (10):2977-82. [ Özet ]
24) Gould VE, Martinez-Lacabe V, Virtanen I, Sahlin KM, Schwartz MM. Differential distribution of tenascin and cellular fibronectins in acute and chronic renal allograft rejection. Lab Invest 1992;67:71-9. [ Özet ]
25) Kawakatsu H, Shiurba R, Obara M, Hiraiwa H, Kusakabe M, Sakakura T. Human carcinoma cells synthesize and secrete tenascin in vitro. Jpn J Cancer Res 1992;83:1073-80. [ Özet ]
26) Mackie EJ, Scott-Burden T, Hahn AW, Kern F, Bernhardt J, Regenass S, et al. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol 1992;141:377-88. [ Özet ]
27) Jones FS, Hoffman S, Cunningham BA, Edelman GM. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989;86:1905-9. [ Özet ]
28) Nakamura Y, Yamamoto M, Sakamoto K, Ohta K, Umeda A, Tsukamoto T, et al. Growth factors, extracellular matrix components and cell adhesion molecules Warthin's tumor. J Oral Pathol Med 2001;30:290-5. [ Özet ]
29) Erickson HP, Lightner VA. Hexabrachion protein (tenascin, cytotaktin, brachionectin) in connective tissue embryonic brain and tumors. Adv cell biol 1988;2:55-90.
30) Dardick I. Histogenesis and morphogenesis of salivary gland neoplasms In: Elis GL, Auclair PL, Gnepp DR editors. Surgical pathology of salivary glands. Philadephia, WB Saunders Company, 1991.p.108-128.
31) Mills SE, Cooper PH. An ultrastructural study of cartilaginous zones and surrounding epithelium in mixed tumors of salivary glands and skin. Lab Invest. 1981;44(1):6-12. [ Özet ]
32) Thesleff I, Mackie E, Vainio S, Chiquet-Ehrismann R. Changes in the distribution of tenascin during tooth development. Development. 1987;101:289-96. [ Özet ]
33) Shibutani T, Iwayama Y, Tsubone M, Ando C, Yamada K, Mori M. Immunohistochemical localization of glycosaminoglycans with the use of monoclonal antibodies in salivary pleomorphic adenomas. Anticancer Res 1990;10:1533-42. [ Özet ]
34) Huvas AG, Paulina AFG. Salivary glands. In: Mills SE, Carter D, Greenson JK, Obermann HA, Reuter V, Stoler MH editors Sternberg’s Surgical Pathology (4. ed) Philadelphia LIPPINCOTT WILLIAMS & WILKINS 2004 p:933-962.
35) Shintani S, Alcalde RE, Matsumura T, Terakado N. Extracellular matrices expression in invasion area of adenoid cystic carcinoma of salivary glands. Cancer Lett 1997;116:9-14. [ Özet ]




